To Issue 142
Citation: Smith S, Scanlan E, Colavita P, “Pro-Tects – A Novel Solution to the Challenge of Biologic Instability”. ONdrugDelivery, Issue 142 (Feb 2023), pp 84–88.
Shane Smith, Eoin Scanlan and Paula Colavita discuss the difficulty inherent in developing parenteral biologic products due to the challenge of creating a stable formulation in the face of surface-mediated protein aggregation and introduce Pro-Tects – Glycome BioPharma’s patented surface-treatment technology platform that offers a solution to this critical issue without introducing further development challenges.
“Stabilisation issues have been known to result in project delays of over a year, with approximately 60% of respondents to a survey by Informa Pharma Intelligence reporting that such issues lead to significant delays, or even outright failure, of projects.”
Biologic therapeutics are one of the fastest growing and most exciting areas in the modern pharmaceutical industry. This category of medicines is made up of large protein molecules, including monoclonal antibodies, and shows great promise in several disease areas, particularly oncology and autoimmune diseases. However, developing, formulating and delivering biologics is far from a trivial task, and several challenges remain to be overcome.
At present, the most common delivery method for biologics is the parenteral route; biologics are inherently delicate molecules, and so parenteral delivery is a natural choice to ensure that they are delivered intact. Specifically, many biologic developers are looking to prefilled syringes (PFSs) as the delivery device of choice, as they are convenient, avoid the need for manual filling required by vials and enable drug developers to design for at-home delivery – another major trend in the pharmaceutical industry.
However, PFSs are not without their own design challenges. Biologic drugs tend to either be large-volume or highly viscous – or both – when formulated for parenteral delivery. However, biologics are frequently subject to protein aggregation when formulated in this way, especially in glass syringes coated with silicone oil for lubricity. The challenge of developing a biologic formulation that remains stable is a critical concern for the pharmaceutical industry; solving it could be a key to unlocking the enormous potential of biologics.
“Glycome BioPharma’s technology enables a swifter time to market for drug development, increasing product competitiveness, and counteracts protein aggregation, improving compliance with safety and efficacy standards.”
THE CHALLENGE OF STABILITY
The struggle to stabilise biologic formulations for parenteral delivery is a consistent factor in drug development project delays and setbacks. Stabilisation issues have been known to result in project delays of over a year, with approximately 60% of respondents to a survey by Informa Pharma Intelligence (London, UK) reporting that such issues lead to significant delays, or even outright failure, of projects.1
The simple fact is that the longer a biologic drug development programme continues, it becomes increasingly expensive and decreasingly competitive, making these delays a major concern for the industry. Getting to market quickly can be a significant factor in the success of a project, especially if a competitor might get there first. The order in which products enter the market matters, with analysis data suggesting that the first and second products to enter the market typically obtain 70% of the overall market share between them.2
Therefore, it is evident that a solution to biologic stability for PFSs will be necessary to accelerate development timelines and achieve a desirable return on investment, as well as mitigate the potential safety concerns associated with protein aggregation.3 The evidence suggests that one of the most significant causes of instability of biologics is surface-mediated aggregation – the interaction between the container materials, such as the silicone oil used in glass syringes, and the drug molecules.4–6 As such, technologies looking to solve the stability problem focus on preventing these interactions.
One of the proposed solutions to the problem of stability is to circumvent the need for silicone oil by switching to cyclic-olefin polymer (COP) syringes.7 There has been some uptake of COP PFSs, but there is hesitancy in the industry when it comes to switching away from glass. Glass is a well understood material, and the conservative nature of the pharmaceutical industry leads it to prefer working with the established over the new, especially where patient safety is concerned. This is true for plastic syringes, which may lead to aggregate generation when put under mechanical stress.8
To continue working with established glass syringes, other biologic drug developers are turning to alternative formulation technologies, such as adding surfactants. This is also not an ideal solution, as biologic formulations are already highly complex, and many are pushing the boundaries of acceptable volume and viscosity for parenteral delivery. As such, surfactants and similar technologies are challenging to work with and may result in their own development delays, ultimately failing to solve the problem at hand, simply replacing one cause for delay with another.4
This leaves an urgent and unmet need for a stabilising technology that works with glass and is simple to implement, working with existing pharmaceutical practices and not contributing to further formulation complexity and production delays. As an expert in the field, Glycome BioPharma has met this need with its patented first generation stabilisation platform for parenteral devices – Pro-Tects.
PRO-TECTS – THE GLYCOME BIOPHARMA SOLUTION
Glycome BioPharma has developed the patented Pro-Tects technology platform to provide intrinsic stabilisation to biologics in parenteral containers. Pro-Tects is a surface treatment for glass and olefin polymer parenteral containers that solves the problem of stabilising biologic molecules by preventing surface-mediated aggregation of the proteins.4
The Pro-Tects technology mitigates protein aggregation by denying the proteins sites where they can anchor to the surface of the syringe. The technology works by covalently binding immobilised sugars to the container-surface interface, thus blocking the protein aggregation mechanism and mitigating the traditional issues with using sugars as a stabilising agent.4 Tests by Glycome BioPharma have demonstrated that this method is hugely successful at reducing aggregation and boosting the stability of biologics in containers using the Pro-Tects platform.
Glycome BioPharma drew the inspiration for using sugars to stabilise biologic drugs from the glycocalyx – the extracellular region of the cell membrane. The glycocalyx is a gel-like mesh containing glycoproteins and glycolipids that contribute to the steric repulsion that prevents undesirable objects from entering the cell. By basing its technology off this naturally occurring barrier, Glycome BioPharma has created a system that effectively and reliably mitigates the ability of proteins to aggregate by interacting with the container surface.
In offering a reliable way to stabilise parenteral biologic formulations, Pro-Tects alleviates the burden of complex formulation on drug development programmes. Glycome BioPharma’s technology enables a swifter time to market for drug development, increasing product competitiveness, and counteracts protein aggregation, improving compliance with safety and efficacy standards.9
PRO-TECTS COMPARISON WITH UNTREATED GLASS
To establish the efficacy of the Pro-Tects solution, Glycome BioPharma conducted a series of tests that compared glass treated with Pro-Tects technology with regular, commercially available glass, including in glass syringes. The results demonstrated that the use of Pro-Tects overwhelmingly reduced protein aggregation at the container-surface interface and confirmed Pro-Tects’s value in contributing to biologic formulation stability.
BSA Protein Rejection on Glass Coupons
First, Glycome BioPharma compared the level of protein adsorption and aggregation of a model protein – bovine serum albumin conjugated with fluorescein isothiocyanate (BSA-FITC) – in untreated glass coupons with those treated with the Pro-Tects platform. Both the Pro-Tects-treated and untreated glass coupons were exposed to a solution of 2 mg/mL BSA-FITC. The glass coupons functionalised with Pro-Tects achieved an almost quantitative reduction in adsorbed protein, with the coated glass demonstrating a protein rejection of 98% (Figure 1).
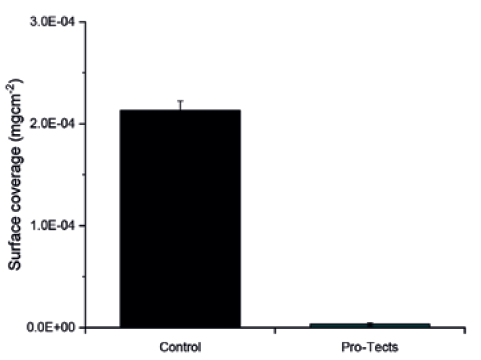
Figure 1: Type I borosilicate glass coupons, untreated (control) and functionalised with Pro-Tects, exposed to a 2 mg/mL solution of BSA-FITC. The amount of protein adsorbed on the surface of each coupon was measured by photoluminescence and normalised by the surface area exposed to it.
BSA Protein Rejection on Glass Syringes
Next, Glycome BioPharma performed a similar test with commercially available glass syringes. As with the glass coupons, the syringes treated with Pro-Tects achieved an almost quantitative reduction in protein adsorption of the model BSA-FITC protein compared with those of untreated glass after contact with a 2 mg/mL BSA-FITC solution. In the case of syringes, the Pro-Tects-treated glass demonstrated a protein rejection of 97% (Figure 2).
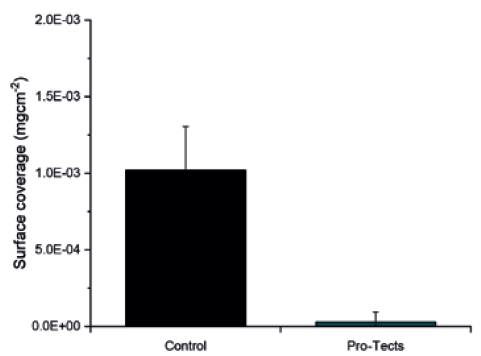
Figure 2: Type I borosilicate glass syringes, untreated (control) and functionalised with Pro-Tects, exposed to a 2 mg/mL solution of BSA-FITC. The amount of protein adsorbed on the surface of each syringe was measured by photoluminescence and normalised by the surface area exposed to it.
Protein Rejection on Glass QCM Sensors
To further investigate the ability of the Pro-Tects platform to mitigate protein adsorption, Glycome BioPharma used quartz crystal microbalance (QCM) technology. QCM is an extremely sensitive mass balance that is able to detect changes in mass per unit area on a nanogram to microgram scale by monitoring changes in frequency as the quartz oscillates under an applied voltage.
Using Type I borosilicate glass QCM sensors, Glycome BioPharma was able to compare the mass of both Pro-Tects-treated and untreated glass after contact with a 2 mg/mL solution of BSA to determine the mass of protein that had adsorbed to the glass. As anticipated, the glass treated with Pro-Tects showed a significant reduction in mass compared with the untreated glass. In this test, the Pro-Tects-treated glass achieved a final protein rejection of 72% (Figure 3).
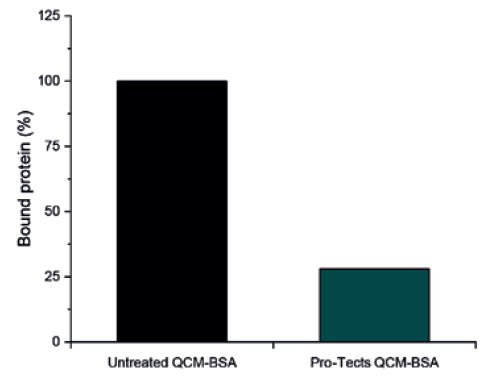
Figure 3: Type I borosilicate glass QCM sensors, untreated (control) and functionalised with Pro-Tects, were exposed to a 2 mg/mL solution of BSA. The binding of protein mass at the surface was measured and reported here as the amount of protein bound to the surface as a percentage, with the mass bound to the control assumed to be 100%.
A subsequent test investigated the mass of Type I borosilicate glass QCM sensors after contact with a solution of 2 mg/mL human immunoglobin G (IgG). As with BSA, glass treated with Pro-Tects demonstrated a significant decrease in bound IgG compared with untreated glass. For the IgG test, the Pro-Tects-treated glass achieved a final protein rejection of 54% (Figure 4).
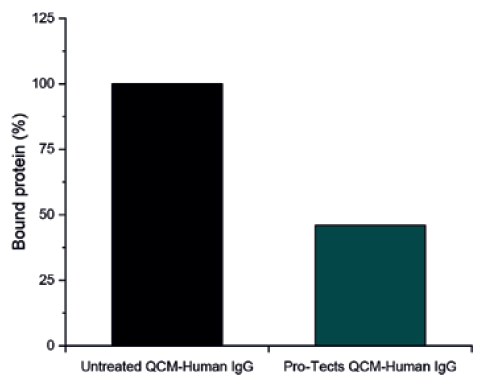
Figure 4: Type I borosilicate glass QCM sensors, untreated (control) and functionalised with Pro-Tects, were exposed to a 2 mg/mL solution of Human IgG. The binding of protein mass at the surface was measured and reported here as the amount of protein bound to the surface as a percentage, with the mass bound to the control assumed to be 100%.
“Pro-Tects has no detrimental effect on syringe glide force and remains robust under a variety of adverse environmental stresses,
including temperature, pH and sterilisation.”
CONCLUSION
Surface-mediated aggregation of proteins is a critical factor when it comes to the stability of biologics. Stability is one of the critical challenges in biologic drug development, potentially creating additional risk both to drug efficacy and project timelines, leading biologics that struggle with stability to become less competitive. Therefore, any biologic development project looking to gain an edge needs an answer to the challenge of stability.
Glycome BioPharma has provided this answer with its patented Pro-Tects technology platform, an immobilised sugar treatment for glass syringes. As discussed in this article, Pro- Tects has demonstrated a significant reduction in bound proteins in both FITC and QCM sensor testing (Figure 5). Furthermore, according to Glycome BioPharma’s internal data, Pro-Tects has no detrimental effect on syringe glide force and remains robust under a variety of adverse environmental stresses, including temperature, pH and sterilisation, which will be discussed in a future article.
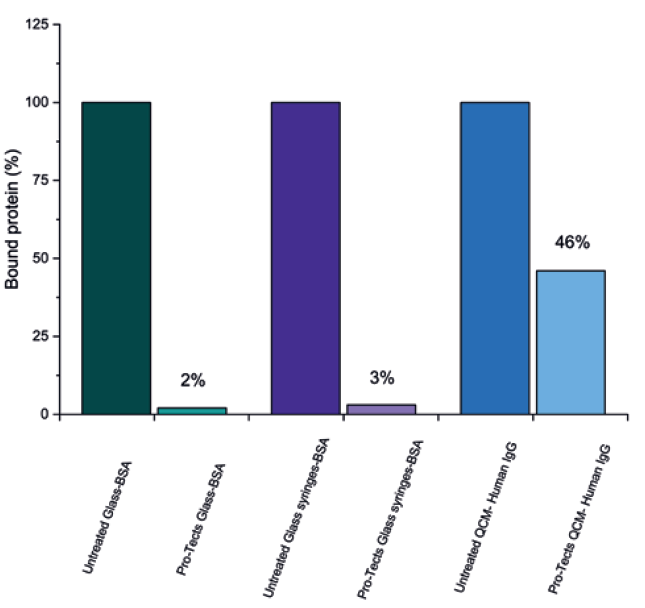
Figure 5: Type I borosilicate glass coupons and syringes, untreated (control) and functionalised with Pro-Tects, exposed to a solution of BSA-FITC alongside Type I borosilicate glass QCM sensors, untreated (control) and functionalised with Pro-Tects, exposed to a solution of Human IgG. The binding of protein mass at the surface was measured and reported here as the amount of protein bound to the surface as a percentage, with the mass bound to the control assumed to be 100%.
Partnering with Glycome BioPharma will enable biopharmaceutical developers to accelerate and de-risk their drug development projects by implementing this exciting and novel technology, with no need to transition away from established and readily available glass syringes, or take on the formulation challenge of including surfactants and thereby increasing risk to their project from another angle. The Pro-Tects technology platform offers a reliable way to tackle the challenge of biologic stability without introducing additional production challenges, improving compliance with safety and efficacy and smoothing the way to market.
REFERNCES
- Schwab B et al, “Early Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase Significantly”. BioProcess International, Mar 2019.
- Kim S, “Technologies and tactics for accelerating end-to-end biologic drug development”. European Pharmaceutical Review, Nov 2021.
- Kotarek J et al, “Subvisible Particle Content, Formulation, and Dose of an Erythropoietin Peptide Mimetic Product Are Associated With Severe Adverse Postmarketing Events”. J Pharm Sci, 2016, Vol 105(3), pp 1023–1027.
- Smith S, Scanlan E, Colavita P, “Surface-Mediated Aggregation – Control of the Liquid-Solid Interfacial Stress”. ONdrugDelivery, Issue 117 (Mar 2021), pp 46–50.
- Perevozchikova T et al, “Protein Adsorption, Desorption, and Aggregation Mediated by Solid–Liquid Interfaces”. J Pharm Sci, 2015, Vol 104(6), pp 1946–1959.
- Mejadnik MR et al, “Postproduction Handling and Administration of Protein Pharmaceuticals and Potential Instability Issues”. J Pharm Sci, 2018, Vol 107(8), pp 2013–2019.
- Waxman L, Vilivalam VD, “A Comparison of Protein Stability in Prefillable Syringes Made of Glass and Plastic”. PDA J Pharm Sci Technol, 2017, Vol 71(6), pp 462–477.
- Kim AK, Kim DJ, Jeong SH, “Do not flick or drop off-label use plastic syringes in handling therapeutic proteins before administration”. Int J Pharm, 2020, Vol 587, Article 119704.
- “Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products”. US FDA, Aug 2014.

