Citation: Mayer T, “Repositioning From IV to Vial-Based SC for Large-Volume Biologics”. ONdrugDelivery, Issue 124 (Sep 2021), pp 42–45.
Thomas Mayer discusses the growing trend of moving away from intravenous drugs administered in clinical and hospital settings to at-home, self-administered vial-based subcutaneous delivery for cost savings, better lifecycle management and improved care.
“The concept of lifecycle management is becoming a key component of drug product management.”
The covid-19 pandemic has highlighted a weak spot in drug delivery – care for patients with chronic conditions can be easily affected, as many have chosen not to visit healthcare settings during the crisis. A March 25, 2020 survey by the American Cancer Society Cancer Action Network found that 27% of cancer patients in active treatment experienced some delay in treatment associated with covid-19.1 This has led drug delivery manufacturers to seek alternatives, such as transitioning from intravenous (IV) drugs given in clinics and hospitals to at-home, self-administered vial based subcutaneous (SC) delivery for cost savings, better lifecycle management and improved care.
IV-to-SC drug repositioning is the strategy of positioning therapies currently requiring IV infusion by a qualified clinician as SC injectables in prefilled devices that are safe and efficacious when self-administered by the patient. Recent advances in the development of anti-cancer medicines in the form of SC drugs could be key to pandemic-era attempts to minimise cancer patients’ exposure to coronavirus during active treatment.
As the pace of competition increases within the pharmaceutical and biotech industries, the concept of lifecycle management is becoming a key component of drug product management. While reformulation is an important approach, efforts to prolong IP benefits have only recently involved IV-to-SC drug re-engineering. This migration is now becoming a significant pathway in the lifecycle of many parenteral drugs. A number of technology approaches are currently being employed to accomplish this migration. By pursuing IV-to-SC strategies, drug patent holders are finding they can achieve a number of competitive advantages.2
For companies with IV products in their portfolio, a transition to an SC formulation can make sense as measures of lifecycle management and differentiation. In other instances, large-volume injection might be advantageous from a pharmacokinetic point of view.
A VIAL-COMPATIBLE DEVICE MEANS A FASTER TRACK TO MARKET
Pharma can ease into the transition from IV to SC delivery by moving to a vial-based solution. Vials remain the container of choice for pharma because most companies have vial-filling capabilities. In fact, 90–95% of liquid drug formulations rely on vials as the primary container. As such, vials are easier to adopt from a drug stability and compatibility perspective.
A large-volume, vial-based SC solution like the LVI-V20 also enables a faster time to market as it is well suited to move from clinical trials to commercialisation. LVI-V20 features an internal reservoir and, with the help of a standard vial adapter, automatically transfers the contents of the vial into the device prior to injection. A temporary drug container inside the device is designed for short-term storage. This device is suitable for both clinical trials and commercial applications, depending on the use case. The LVI-V20 is designed for operation with standard vials up to 20 mL.
The advantage of a vial-compatible solution is the relative ease of integration into existing and proven pharma processes. Pharma companies can keep using existing primary containers and do not have to develop a specific container and create stability data.
Additionally, LVI-V20 provides flexibility from a payload point of view, able to be filled from 1–20 mL. Often, in early development phases, it’s unclear what the final dose volume will need to be, so something that provides more headroom offers more flexibility to the overall process. LVI-V20 offers the ability to go to clinic without having final dose volume determined and, because it can be filled with less than 20 mL, provides pharma companies with a great deal of flexibility.
Ideally, a pharma company would start with the LVI vial-based solution in clinical trials or early commercial development and then, in cases where home use or self-care is intended, transition to a solution with a prefilled and preassembled cartridge, such as the Sonceboz LVI-P.
“The advantage of a vial-compatible solution is the relative ease of integration into existing and proven pharma processes.”
SUBCUTANEOUS DELIVERY EMPOWERS PATIENTS
An increasing number of people with chronic diseases, along with the availability of self-administered drug delivery devices, have propelled the growth of global subcutaneous drug delivery devices market – valued at US$22.4 billion (£16.3 billion) in 2019 and expected to reach $71 billion by 2030.3 SC drug delivery refers to the medication being injected in the SC layer (between the skin and muscle) of an individual. These injections are less invasive than IV and, in many cases, manageable by patients themselves.
Depending on the drug, SC drug delivery devices make it possible for patients with diabetes, cancer, autoimmune diseases or rare diseases to self-medicate anywhere and at any time without professional help. SC drug administration has proved to be safe, well-tolerated, effective – and often preferred by healthcare providers and patients, resulting in optimised drug delivery costs.4
Traditional SC injection devices, such as prefilled syringes or autoinjectors, are limited to a maximum delivery volume of 3 mL. Strategies for overcoming this problem are crucial to widening the scope of drugs that may be administered subcutaneously.
To achieve IV-equivalent efficacy, SC formulations often require higher volumes or higher concentrations – in some cases, a combination of both. To meet this challenge, Sonceboz developed the LVI-V20 as a vial-compatible solution and LVI-P20 as a prefilled, preloaded cartridge solution, both of which can handle drug payloads up to 20 mL (Figure 1). The LVI-P acts similarly to an autoinjector: the device is placed on the skin and activated by the touch of a single button – the remaining steps, such as placing the injection cannula and initiating the injection, are done by the device and the user is informed about the current state of operation, including when the procedure is completed and the full intended dose received. This process is monitored by a series of embedded sensors.
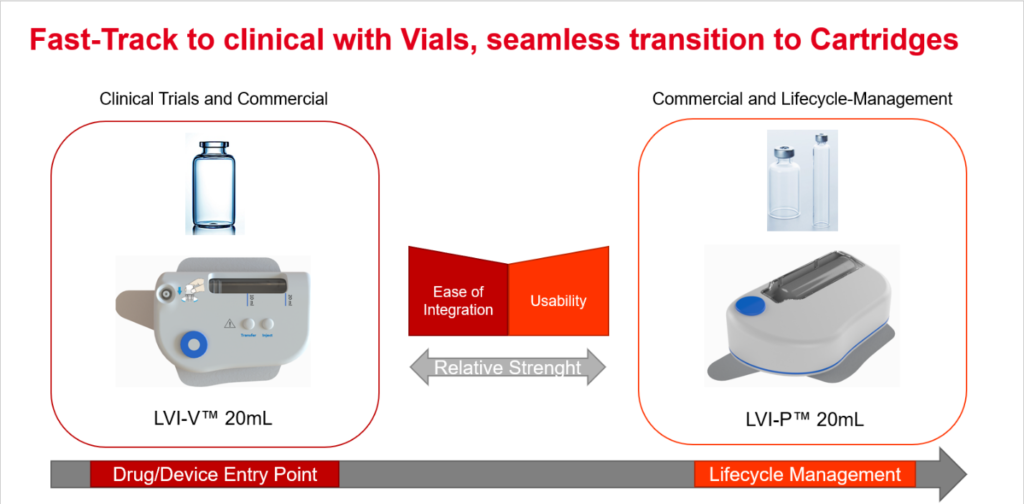
Figure 1: Starting with a vial-based product provides both a fast-track to clinical trials and a seamless transition to a cartridge-based solution for at-home use.
“Empowering the patient with a wearable injector to be used at home eases administration, improves compliance and eliminates costly healthcare visits.”
WEARABLE, SMART SC INJECTORS
The rise in the number of biologic drugs, such as monoclonal antibodies, has increased the desire for and awareness of injection devices in general. While the market today is dominated by devices like autoinjectors, higher dosage volumes and viscous formulations of certain drugs are further emphasising the need for alternative injection methods, including wearable injectors. This is often due to reformulation efforts, where a trade-off between efficacy and drug stability must be found.
Empowering the patient with a wearable injector to be used at home eases administration, improves compliance and eliminates costly healthcare visits. Aside from the home-use scenario, devices like the LVI-V20 can also improve care in injection clinics or hospitals, since the automated injection/infusion process allows for higher patient throughput in a less invasive fashion. Done right, this can have a substantial impact through healthcare cost reduction.
The increasing prevalence of diabetes, chronic pain, cancer and autoimmune diseases, such as rheumatoid arthritis, is driving the use of wearable injectors. This is expected to create a lucrative opportunity for advanced drug delivery device manufacturers to make wearable devices for administering larger dose volumes or enabling self-medication in the comfort of a patient’s home. This is being witnessed by a global market for wearable injectors estimated at $4.8 billion in 2020, and that could reach upwards of $18.3 billion by 2028.5
The Sonceboz wearable injector platform (Figure 2) comes with built-in connectivity using Bluetooth Low Energy as the communications standard. Information such as time and volume of delivered dose can be transmitted to relevant stakeholders such as healthcare providers. If the device simplifies the overall treatment experience, it will most likely have a positive impact on compliance. Thanks to connectivity, the device provides feedback to the patient that the treatment regimen has been followed correctly. Pairing this information with elements of gamification can most likely improve compliance and thereby help solve one big challenge in healthcare – noncompliance. Thus, in the coming years, the adoption of connected device technology is expected to accelerate significantly.
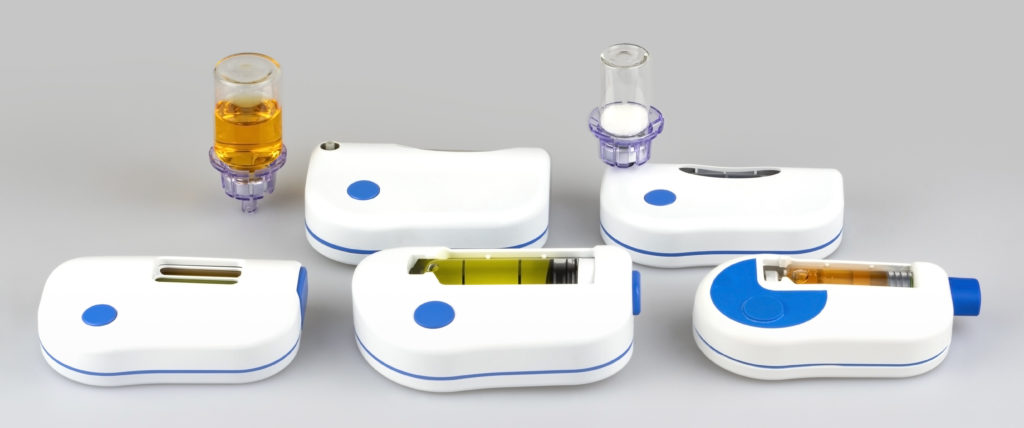
Figure 2: The Sonceboz wearable injector platform offers different delivery volumes, programmable pump delivery profiles and container options.
“While vials are the go-to container for large-volume drugs, they are not ideal for wearable devices use due to their non-compressible/ collapsible nature.”
While vials are the go-to container for large-volume drugs, they are not ideal for wearable devices use due to their non compressible/ collapsible nature. This means that, for successful drug retrieval, one needs to manage vial orientation to avoid incomplete injection. There are different approaches to this challenge. One is to monitor orientation with digital orientation sensors – another is to first transfer the drug into a collapsible container. Sonceboz follows the latter approach since it believes this is the only way to guarantee that the full dose of drug is delivered every time. While there are devices that need to be aligned on the body in a certain way so that the pump can access the drug, Sonceboz’s devices pump drug regardless of the spatial orientation of the device on the skin – whether upside down, sideways or tilted.
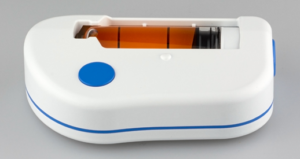
Figure 3: The large volume injector (LVI™).
The Sonceboz wearable SC devices, like the LVI (Figure 3), are designed with an electronic drive system which can be programmed to give pharma and healthcare providers options as to how the drug will actually be delivered. The pump system is independent of the drug container so one can connect both small and very large containers. Additionally, the pump is constructed with materials and coatings that are compatible with large-molecule biologics.
Whether a drug manufacturer chooses a vial- or prefilled cartridge-based SC delivery device, improved patient care, options for lifecycle management and potential cost savings are potential benefits of reformulating an IV-based delivery method. While the past 10 years have been fairly slow, the next 10 will be clearly different, with many active programmes for drug-device combinations in development and many programmes of IV-to- SC reformulation underway. IV to SC is where Sonceboz’s technology will have an impact.
REFERENCES
- “COVID-19 Healthcare Delivery Impacts”. Tracie Healthcare Emergency Preparedness Information Gateway, Apr 12, 2021.
- “Intravenous-to-Subcutaneous (IV-to-SC) Drug Migration, 2019-2024”. Research Report, ResearchAndMarkets.com, March 20, 2019.
- “Subcutaneous Drug Delivery Devices Market Is Expected to Generate Revenue Worth US$ 71 billion by 2030, Globally”. insightSLICE, Nov 17, 2020.
- Bittner B et al, “Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities”. BioDrugs, 2018, Vol 32(5), pp 425–440.
- “Wearable Injectors Market Size Worth $18.3 Billion By 2028 | CAGR: 17.2%”. Grand View Research, Mar 24, 2021.

