Citation: Pond G, “Smarter Medicines to Secure Products and Promote Patient Engagement”. ONdrugDelivery, Issue 122 (Jul 2021), pp 32–36.
Gary Pond looks at digital authentication and discusses how on-dose microtaggants can provide a high level of security in authenticating tablets and capsules in the fight against counterfeit products.
COLORCON’S ON-DOSE AUTHENTICATION SYSTEM PROVIDES A DIGITAL SECURITY LAYER TO SAFEGUARD PATIENTS AND UPHOLD BRAND INTEGRITY
Take Control with SoteriaRx® Digital Authentication
“Perhaps the most significant benefit of on-dose authentication is to increase the speed of decision making in the supply chain for suspected counterfeit or illegally diverted drugs.”
Pharmaceutical companies are accountable for ensuring that their medicine is safe when it arrives in the hands of patients. The covid-19 pandemic has led to a dramatic increase in the number of online pharmacies and the supply of counterfeit drugs. As criminal gangs employ more sophisticated methods, sometimes re-using real packaging, smarter medicines, which protect patients, are of paramount importance for brand integrity and trust. On-dose authentication solutions are difficult to replicate or reverse engineer and provide a robust and reliable means of deterring and identifying counterfeiting and verifying product identity. Perhaps the most significant benefit of on-dose authentication is to increase the speed of decision making in the supply chain for suspected counterfeit or illegally diverted drugs. When a suspect event occurs, pharma companies will be able to react faster to determine if the product is real or fake, regardless of whether they have access to the packaging. Sometimes this process can take days or weeks but, with on-dose authentication, it is possible to authenticate a product in real time.
Colorcon, a world leader in the development and supply of film-coating systems and excipients for the pharmaceutical industry, now provides on-dose authentication technologies to meet client needs. The SoteriaRx® on-dose authentication platform provides the most robust technology currently available for instant verification of drugs at the dosage level.
In addition to desktop and mobile readers, proof of concept shows that it is now possible to identify the on-dose microtaggants using a smartphone app. This will enable the ability to improve patient outcomes by reminding patients to take their medication, scan the tablet to verify that the right medicine is being taken at the right time, provide patient support materials and alert carers if vulnerable patients fail to take their medicines. This technology can also play an important role in supporting virtual clinical trials.
THE THREAT OF ILLEGAL ONLINE PHARMACIES
Globalisation and outsourcing have led to complex supply chains for many pharmaceutical products. This increases the risk of counterfeiting and diversion, which can result in health risks and loss of trust for the consumer, impact revenues and cause reputational damage to the brand owner.
Many countries have introduced serialisation legislation that requires product identifiers to be used on each package to provide traceability throughout the distribution supply chain. However, traceability and security measures focused on the packaging level may still not be enough to protect patients. Even if a package is authentic, it is impossible to verify whether the medicine inside is real, fake or has been diverted.
With the growth in online sales, virtual pharmacies are a major contributor to the supply of counterfeit medicines. When looking to buy medicine online, it has been found that 100% of searches return links for illegal pharmacies,1 over 90% of online pharmacies operate illegally, around 62% of medicines purchased online are fake or substandard2 and over 90% of incidents are in the highest risk category, potentially endangering life.3 In 2018, the Pharmaceutical Security Institute reported that counterfeit medicine incidents increased by 33% in a single year and 138 countries were impacted.4
Since the start of the covid-19 pandemic, there has been a massive increase in online drug sales. A record number of fake online pharmacies were shut down in May 2020 as criminals sought to take advantage of the crisis. Interpol led a global crackdown that saw more than 100,000 online marketplaces offering illicit drugs removed. Between May 18 and 25, there were 277 arrests in 92 countries.5 In the UK, fake medicines worth more than US$13 million (£9.17 million) were seized as part of the operation.
In 2020, the WHO identified the issue of counterfeit drugs as one of the urgent health challenges for the next decade.6 Writing in the American Journal of Tropical Medicine and Hygiene in 2019, doctors from the US government, universities, hospitals and the pharmaceutical company Pfizer warned that the rise in “falsified and substandard medicines” had become a “public health emergency” and that poor quality drugs exact an annual economic toll of up to $200 billion.7 In addition to the direct harm that they cause, the supply of counterfeit antibiotics is a major driver of antimicrobial resistance.
HOW THE INDUSTRY CAN FIGHT BACK – MAKING MEDICINES SMARTER WITH ON-DOSE MICROTAGGANTS
“Microtaggants are uniquely encoded materials that are virtually impossible to replicate or reverse engineer. They can be incorporated in tablet coatings or in the inks used on tablets or capsules and detected in the lab or the field using handheld devices.”
There is growing recognition that packaging and serialisation will not solve the problem of counterfeit medicines. As serialisation matures, it will provide a valuable tool for track and trace but, for the highest risk products, on-dose microtaggants provide a much higher level of security, allowing individual tablets and capsules to be authenticated. Instant verification at the dosage level reduces the risk of counterfeiting and product diversion while facilitating quality control and returns monitoring. Figure 1 shows a range of security measures that are now available.
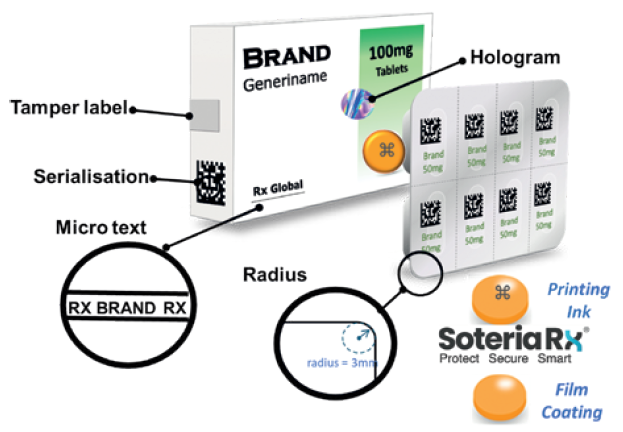
Figure 1: Multi-layered security approach for pharmaceutical products.
Appropriate security measures will be determined by the risks associated with each product for different stakeholders (Figure 2). For low-risk products, serialisation on the secondary packaging may provide adequate protection but, for high-risk drugs, on-dose authentication using microtaggants is required to better protect patients and brands.
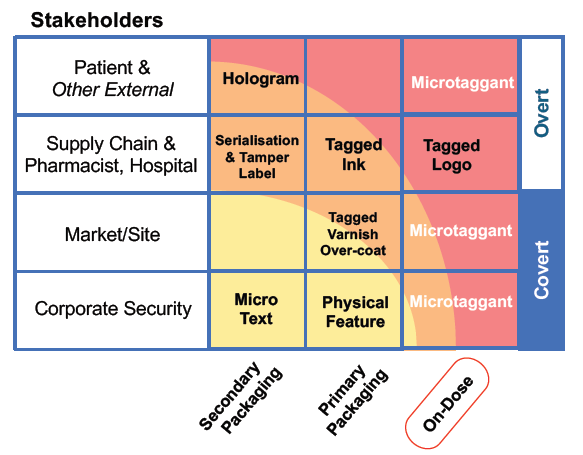
Figure 2: Stakeholder security measures for pharmaceutical products.
Microtaggants are uniquely encoded materials that are virtually impossible to replicate or reverse engineer. They can be incorporated in tablet coatings or in the inks used on tablets or capsules and detected in the lab or the field using handheld devices. The technology requires no additional manufacturing equipment or capital investments, and the US FDA has stated that, when microtaggants are pharmaceutically inactive and incorporated into new or existing drugs, they can be treated as excipients without the need for further clinical trials. This means that manufacturers of an approved product in the US would only need to incorporate the microtaggants as a Level 1 post-approval change in the Annual Report. For NDAs, inclusion would be part of the standard submission process.
“The introduction of a smartphone app means that patients can play a part in verifying their medication, and that interaction could be leveraged to add value through patient engagement and brand loyalty.”
The quantities of microtaggants added to film coatings or inks are so small their use has no impact on how coatings are applied, and they will not affect the product’s stability, disintegration or dissolution. The cost per tablet of incorporating the microtaggants is negligible relative to other manufacturing costs as the microtaggants are simply included in the standard film coating or tablet printing process.
Colorcon has the exclusive worldwide rights to molecular taggants from Applied DNA Sciences (Stony Brook, NY, US) and TruTag Technologies’ (Emeryville, CA, US) silica technology for on-dosage use, and both are marketed under the SoteriaRx solution platform.
These different types of microtaggants are now commercially available and can be customised with unique information for product verification and traceability.
DNA Microtaggants
Non-biologic molecular DNA is robust and easy to detect and, because it is possible to produce different versions of the same DNA molecule, it can be made regional-, product- or company-specific. The microtaggants are simply added to the standard tablet film coating or capsule printing process and can then be detected using appropriate reagents (such as an antigen and antibody). The microtaggants are not damaged by exposure to heat and pressure during the coating process, and the integrity of the DNA remains consistent throughout the shelf life of the product.
Silica Microtaggants
Spectrally encoded silica microtaggants can be detected by how they reflect light. Like DNA, the microtaggants are incorporated into the film coating or printing ink and applied during the manufacturing process. Silica is already widely used as a pharmaceutical excipient within tablet and capsule formulations, making it an easy material to include.
Recent work has concentrated on developing convenient readers to scan information carried on the microtaggants. Proof of concept demonstrates that tablets or capsules with silica microtaggants can be identified using a smartphone app, providing instant verification by internal and external quality assurance teams, including enforcement agencies.

Figure 3: Smartphone authentication app.
SMARTPHONE AUTHENTICATION APPS ENABLE DIRECT PATIENT ENGAGEMENT
The introduction of a smartphone app (Figure 3) means that patients can play a part in verifying their medication, and that interaction could be leveraged to add value through patient engagement and brand loyalty. Through software development kits, this type of app can easily be customised for individual companies and specific medications to help:
- Improve adherence and achieve better outcomes for patients It is easy for someone who is sick or confused to take the wrong drugs or miss doses. The app can remind patients when it is time to take their medication and which tablets to take. For many conditions, for example, following an organ transplant or for the treatment of certain cancers, adherence to the correct medication regime is vital. Scanning each tablet before it is taken would reassure the patient, and results could also be relayed to a caregiver or medical team, alerting them if the patient has failed to scan the medication at the correct time.
- Promote patient trust and brand loyalty Cases have been reported of patients with extremely serious conditions failing to take their medication due to concerns over side effects. The app could remind and actively assist patients to recognise certain side effects, provide reassurance that they are not unusual, and help manage these initial challenges to ensure they stay compliant with their regimen. There could also be reminders for repeat prescriptions and updates as new information becomes available. This type of engagement could give the patient a sense that the drug manufacturer is doing all it can to support them.
- Make virtual clinical trials more robust and reduce costs On-dose microtaggants can also be incorporated in clinical supply programmes, to reduce the opportunity for error in the allocation and administration of these expensive trials. Introducing on-dose authentication into clinical trials allows for validated patient engagement at any stage in the trial, ensuring the right patients are taking the right drugs at the right dosage. This validation is all done without impacting the blinding process. Soon it may be possible for patients to film themselves scanning and taking a drug as required for the trial, thus improving visibility and compliance and providing real-time patient support.
CHOOSING THE BEST SOLUTION
“No new investment is needed to incorporate the microtaggants; they are simply added to a standard tablet film coating process.”
It is important to understand how effective and reliable an authentication process will be, and the benefits that may be gained as a trade-off for the time and resources required to implement advanced technology. By leveraging current tablet coatings and print technologies, no new investment is needed to incorporate the microtaggants into standard manufacturing procedures; they are simply added to a standard tablet film coating or capsule printing process. The film-coating process does not change, there is no impact on the final tablet finish and the cost is negligible (Figure 4).
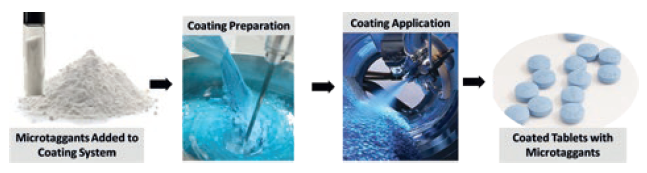
Figure 4. Simple to apply directly on-dose via the tablet coating system.
Machine reading of security features is faster and more reliable than manual inspections and will be suitable for high volume applications. SoteriaRx technology equips product quality teams with a faster way to identify rogue batches and make more informed decisions as to whether a batch needs to be held pending further investigation or immediately recalled.
Multinational Companies are now Implementing Customised On-Dose Authentication for High-Risk or High-Value Products
Incorporating microtaggants directly into the product and using the smartphone app provides a unique opportunity for brands to secure their products and expand this technology to interact with patients for the best possible outcomes. Through real-time data collection and analysis, patient-focused product teams will be able to provide more personalised support resources when needed.
Following extensive R&D by in-house scientists and its technology partners, Colorcon is now working with leading pharmaceutical companies to help them consider how SoteriaRx integrates with their current product security strategies to provide an optimal solution. We do not need to look to the future for smart medicines to become a reality. Innovative technology is available today that enables digital authentication of the patient’s medicine using their smartphone and a secure cloud application to provide realtime support.
REFERENCES
- “Illegal online pharmacies: how endemic are they?”. Pharmaceutical Technology. July 20, 2018. Web Page, https://www.pharmaceuticaltechnology. com/comment/illegalonline- pharmacies-endemic/
- “Trade in Counterfeit Pharmaceutical Products”. PDF, Illicit Trade, OECD and the European Union Intellectual Property Office, 2020.
- “Falsified Medicines”. International Federation of Pharmaceutical and Manufacturers Association. Web Page, https://www.ifpma.org/ topics/falsified-medicines/, accessed June 2021.
- “Measuring Pharma Crime”. Pharmaceutical Security Institute. Web Page, https://www.psi-inc.org/ pharma-crime, accessed June 2021.
- “Pharmaceutical Crime Operations”. Interpol. Web Page, https://www. interpol.int/en/Crimes/Illicit-goods/ Pharmaceutical-crime-operations, accessed June 2021.
- “Substandard and falsified medical products”. World Health Organization. https://www.who. int/health-topics/substandard-andfalsified- medical-products#tab=tab_1, accessed June 2021.
- Nayyar GML et al, “Falsified and Substandard Drugs: Stopping the Pandemic”. Am J Trop Med Hyg, 2019, Vol 100(5), pp 1058–1065.
Previous article
ADDRESSING ONGOING AND NEW BIOAVAILABILITY CHALLENGESNext article
FORMULATION SOLUTION FOR MOISTURE-SENSITIVE DRUGS
