To Issue 158
Citation: Aqrawe Z, Rogueda P, Murnane D, Eng B, “Soft Mist Inhalers Versus Nebulisers: Delivery Equivalence & Future Therapies”. ONdrugDelivery, Issue 158 (Apr 2024), pp 24–28.
Zaid Aqrawe, Philippe Rogueda, Darragh Murnane and Bryan Eng, discuss the opportunity presented by soft mist inhalers as an alternative delivery device to nebulisers for inhaled therapies, contrasting the two device categories across multiple factors.
In exploring the landscape of respiratory drug delivery, the comparison between nebulisers and soft mist inhalers (SMIs) emerges as a subject of keen interest. These devices (Figure 1), pivotal in managing various respiratory conditions, have evolved to meet the diverse needs of patients.1 SMIs, in particular, represent a significant advancement, offering unique benefits over traditional nebulisation therapy. In considering their operational mechanisms, usage and efficacy, it becomes clear that SMIs offer clear advantages in various aspects of pulmonary drug delivery.
Figure 1: Both nebulisers and soft mist inhalers are pivotal in managing various respiratory conditions (not to scale).
“In the world of biologics, prime candidates for making the transition from nebulisers to delivery with an include mRNA, vaccines, oligonucleotides and other therapeutic proteins, some in LNP formulations.”
At the heart of this analysis is the principle of delivery equivalence and the ability to deliver biologics – the therapies of the future – juxtaposed with patient-centric considerations, such as ease of use, environmental impact and cost-effectiveness. While both SMIs and nebulisers aerosolise medication for inhalation, SMIs excel by providing precise, unit-dose delivery with superior lung deposition.2–4 This article delves into the scientific and practical advantages of SMIs, demonstrating their potential to enhance treatment outcomes, patient adherence and overall quality of life for individuals with respiratory conditions.
COMPARING IN VITRO PERFORMANCE
SMIs and nebulisers both aerosolise liquid formulations, with the key difference being that dose delivery for an SMI occurs within seconds, as opposed to minutes for a nebuliser. Taken in tandem with the superior ability of SMIs to achieve deep lung deposition and aerosolise medications with a high fine particle fraction (FPF) (Table 1), it is reasonable to conclude that, if a drug can be formulated as a solution for a nebuliser, it is highly likely that that same drug can be formulated for and delivered by an SMI. Going beyond this, SMIs have the added capability to deliver drugs solubilised in ethanolic solutions or other solubilising media, widening the scope of delivery to molecules that are insoluble in aqueous media.
| Product | Molecule | MMAD (μm) | FPF<5μm (%) |
| MicroBase μSMI | Budesonide | 5.3 ± 0.1 | 44.3 ± 1.7 |
| Aerogen Solo | Budesonide | 5.1 ± 0.3 | 47.3 ± 5.0 |
| Philips Innospire Go | Budesonide | 4.9 ± 0.1 | 50.8 ± 0.9 |
| Pari eRapid | Budesonide | 5.9 ± 0.2 | 35.8 ± 1.9 |
| Respimat | Fenoterol | 4.6 ± 0.1 | 52.0 ± 1.8 |
| Respimat | Tiotropium | 2.7 ± 0.5 | 53.5 ± 6.6 |
Table 1: Median mass aerodynamic diameter (MMAD) and FPF for various marketed vibrating mesh nebulisers (over time) and SMIs (per breath).5–8
An example of the nebuliser to SMI transition is the successful and equivalent aerosolisation of salbutamol nebules with an SMI (MRX004), for which a single breath FPF<5μm of 63.2 ± 2.1% was achieved.9 In the world of biologics, prime candidates for making the transition from nebulisers to delivery with an SMI include messenger ribonucleic acid (mRNA), vaccines, oligonucleotides and other therapeutic vaccines, some in lipid nanoparticle (LNP) formulations. One example of this potential is dornase alfa.
Delivery of Dornase Alfa via an SMI
Dornase alfa is a cystic fibrosis medication delivered via nebuliser and is a clear candidate for delivery via SMI. A study conducted by Merxin Ltd and Intertek10 found that an aqueous solution of dornase alfa, delivered via MRX004, demonstrated a high FPF and only minimal loss of enzymatic activity (Figure 2). Data from tests using a next-generation impactor showed an FPF (<5 μm) of 59.4%. The study concluded that an SMI was capable of successfully and efficiently delivering dornase alfa, with a retention of 90% of the enzymatic activity compared with the nebule.
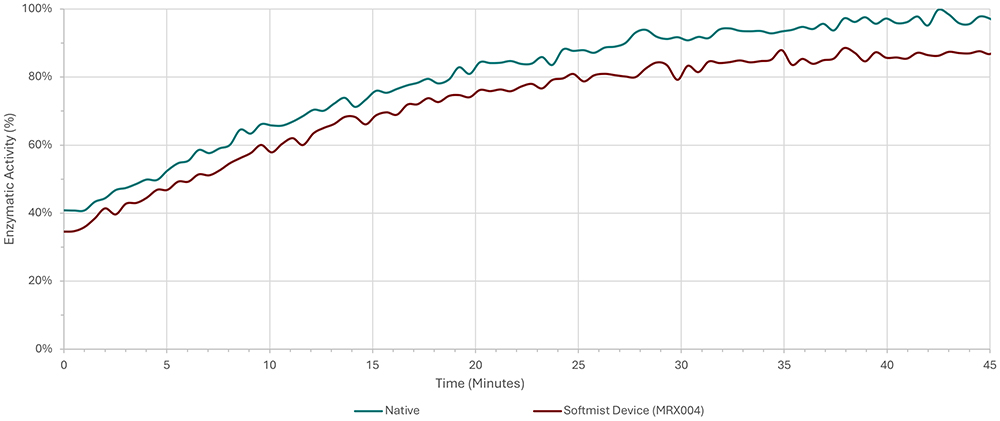
Figure 2: Enzymatic activity of dornase alfa activity prior to delivery and after delivery by the SMI MRX004.
“By adopting the final device as early as possible during the development process, it is possible to largely eliminate the replication of work that is necessary if the device is changed further into the process, during Phase II or even Phase III.”
Aerosolising mRNA & Nanoparticle Formulations
A study conducted by Miao H et al (2023) examined the ability of SMIs and nebulisers to deliver an mRNA-LNP vaccine formulation, and concluded that the SMI compared favourably with the nebuliser technologies in a number of ways.4 The SMI provided a softer method of aerosolisation than nebulisation, with the structure of the LNPs remaining unchanged after delivery with an SMI from jet, vibrating mesh and ultrasonic nebulisers. Laser diffraction of the emitted aerosol showed that the SMI was able to generate a finer aerosol than was seen with a vibrating mesh nebuliser, and so was more suitable for lung delivery. Furthermore, the LNP structures tended to be deconstructed and reassembled by vibrating mesh nebulisation leading to poorer mRNA encapsulation than when aerosolised using an SMI.
Other advantages of aerosolisation using an SMI rather than a nebuliser included a superior entrapment efficiency for the SMI aerosol and greater biological activity of the mRNA after delivery with the SMI. The biological activity was examined by both ex vivo and in vivo fluorescence imaging, which suggested that SMI administration resulted in an mRNA concentration approximately four times greater than administration with a vibrating mesh nebuliser.
PATIENT PREFERENCES
Ease Of Use
In practice, SMIs have proven popular with a wide range of patients, citing increased ease of use and convenience when compared with nebulisers. Three key factors that contribute to this preference are the portability of SMIs, which are of a comparable size to MDIs and DPIs; relative speed and simplicity of operation, requiring four straightforward steps – “open, twist, press/inhale, dose” – with the dose delivered in 10 seconds,1 whereas nebulisers require 14 steps;11 and their significantly lower maintenance requirements, needing only simple and infrequent cleaning, such as washing the mouthpiece once a week, while nebulisers have relatively intensive cleaning requirements, need extensive assembly (chamber, tubing, mask, nasal clip, etc), require parts to be changed out and need the patient or carer to fill the formulation chamber for each use session.
However, it is important to remember that SMIs are not a simple one-size-fits-all replacement for nebulisers – specific patient demographics will still find nebulisers preferable based on their needs. Nebulisers remain the preferred option for paediatrics and geriatrics, where their ability to deliver high doses over an extended period of time, combined with a lack of need to co-ordinate inhalation with activating the device, better suits their needs. On the other hand, more active patients who are regularly mobile, especially outside the home, will find the portability and speed of delivery offered by SMIs is much better suited to their lifestyle. Additionally, the superior deep-lung penetration of SMIs may suit patients who require consistent and effective management of chronic respiratory conditions.
A key deciding factor as to whether a patient demographic is better suited to an SMI or a nebuliser is whether they can master the necessary co-ordination between breath and device activation. Studies suggest, however, that this may not be an onerous ask with proper training and facilitation. For example, it has been shown that children under five years old can use SMIs with near 100% accuracy if a valve holding chamber is employed.12,13
Sustainability
Environmental impact is an increasingly prominent factor in the minds of patients, payers and regulators when considering drug delivery devices. In order to combat climate change, societies worldwide are looking to reduce the carbon footprint of current technologies or replace those currently in use with less environmentally damaging alternatives. Here, SMIs stand out as a favourable alternative to both MDIs and nebulisers, as they require neither propellant nor electricity.
Comparing the carbon footprints of SMIs and nebulisers, SMIs have a noticeable advantage over nebulisers (Table 2). Therefore, drug developers looking to meet their net zero targets would be wise to consider formulating their respiratory medicines as an SMI.
| Product | Carbon Footprint (kg CO2-eq/dose) |
|
| SMI | Tiotropium Respimat (disposable) | 0.013 |
| Ipratropium bromide + fenoterol Respimat | 0.013 | |
| Nebuliser | Albuterol sulfate jet nebuliser | 0.047 |
Table 2: Carbon footprint in CO2 equivalents (CO2-eq) per month for an SMI and nebuliser. All CO2-eq data were standardised for dose to allow comparisons.14,15
Affordability
Cost of ownership is always going to be a key consideration for any drug delivery device. If a medication is overly expensive for a patient, it is possible that there will be a negative effect on adherence, lowering the efficacy of the treatment overall. In a survey conducted by Asthma + Lung UK, patients reported that using their nebuliser during an energy crisis was a point of anxiety.16 SMIs are cheaper than nebulisers on a per-device basis and, as they are purely mechanical as opposed to electronic, have a lower operating cost (Table 3). Furthermore, SMIs may provide a cost saving at the healthcare provider level, due to the lower amount of training required to familiarise a patient with their device.
| Cost Item | Nebuliser | SMI |
| Device cost | £50–£745 | £4–£13 |
| Drug cost/dose | £1.23 per dose (for salbutamol nebuliser solution) |
£0.50 per dose (based on Spiriva Respimat (60 doses) |
| Device parts | £5–£50 | N/A |
| Energy cost | 10–70 kWh per year | N/A |
| Cleaning cost | Energy associated with dishwasher or running water |
N/A |
| Training time | Longer training time needed | Technique mastered quickly, reducing training costs |
Table 3: Comparison of costs associated with SMIs and nebulisers.17–20
BRIDGING PRECLINICAL AND CLINICAL DEVELOPMENT
In current respiratory drug development, nebulisers are the standard during preclinical animal studies,21 due to the control over the dosage and properties of the aerosol. Many development programmes then continue to use a nebuliser as they progress to clinical trials, even if it is not the ideal device for human use nor the anticipated final form of the product, postponing the point at which the final device is decided on. While it may seem initially appealing, this paradigm leads to increased costs down the road as clinical work must be repeated with the new device. This pitfall can be avoided by using an SMI.
Because of the similarities between SMIs and nebulisers, the nebuliser data gathered during the preclinical phase will still be applicable to clinical studies using an SMI – as both devices deliver a solution-based formulation as a soft aerosol, the difference in variables is minimised. This means that the pharmacokinetic and pharmacodynamic preclinical data can be smoothly transitioned into clinical trials with a more patient-friendly and cost-effective device.
Bringing forward the point at which the drug formulation is transitioned to a device intended for commercial launch also offers significant improvements to the clinical trial process, smoothing the pathway from preclinical studies to regulatory submission. By adopting the final device as early as possible during the development process, it is possible to largely eliminate the replication of work that is necessary if the device is changed further along in the development programme, during Phase II or even Phase III. With an SMI, this transition is possible much sooner than it would be with other categories of inhaler, even from as early as the first clinical study. This means getting vital medications to patients sooner and at a lower cost.
These advantages are particularly relevant to biologic therapies. These novel biomolecules are typically synthesised in aqueous media, which means that they can be seamlessly formulated for SMI delivery without the need for expensive and time-consuming reformulation into another medium, as would be the case with other inhaler types. Combined with the fact that SMIs cause significantly less damage to these delicate molecules than nebulisers, it is evident that SMIs are the natural fit for delivering biologics, and transitioning to SMI delivery as early as possible in development will enable developers to get the best out of their drug formulations.
THE SOFT MIST INHALER OPPORTUNITY
There is a clear opportunity in pharmaceutical development to reassess the place of nebulisers in respiratory product development, both at the transition from preclinical to clinical trials and as a commercial device in the hands of patients. SMIs are, for many patients, a more convenient and desirable device that compare favourably with nebulisers across a wide variety of factors and are able to deliver many of the same formulations. Forward-thinking drug developers looking to reduce costs, smooth the development pathway and stand out in the market would be wise to consider the potential benefits that using an SMI could bring to their project.
REFERENCES
- Pleasants RA, Hess DR, “Aerosol Delivery Devices for Obstructive Lung Diseases”. Respir Car, 2018, Vol 63(3), pp 708–733.
- Talwar D et al, “The emerging role of nebulization for maintenance treatment of chronic obstructive pulmonary disease at home”. Lung India, 2021, 38(2), pp 168–173.
- Wang H et al, “Tunable rigidity of PLGA shell-lipid core nanoparticles for enhanced pulmonary siRNA delivery in 2D and 3D lung cancer cell models”. 2024, J Control Release, Vol 366, pp 746–760.
- Miao H et al, “Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA”. Int J Pharm, 2023, Vol 640, Article 123050.
- Hsiao S et al, “A Novel Contact-triggered Vibrating Mesh Nebulizer: Aerodynamic Performance and Drug Distribution of Suspension Drug Delivered with MicroBase μSMI”. RDD 2018, 2018, Vol 2, pp 459–462.
- Pham S et al, “In Vitro Characterization of the eFlow Closed System Nebulizer with Glycopyrrolate Inhalation Solution”. J Aerosol Med Pulm Drug Deliv, 2018, Vol 31(3), pp 162–169.
- Wei X et al, “In Vitro Tests for Aerosol Deposition. VI: Realistic Testing with Different Mouth-Throat Models and In Vitro-In Vivo Correlations for a Dry Powder Inhaler, Metered Dose Inhaler, and Soft Mist Inhaler”. J Aerosol Med Pulm Drug Deliv, 2018, Vol 31(6), pp 358–371.
- Ciciliani A-M, Langguth P, Wachtel H, “In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy”. Int J Chron Obstruct Pulmon Dis, 2017, Vol 12, pp 1565–1577.
- Antoniak D et al, “Transitioning pMDIs to SMIs: salbutamol as a case study”. Poster Presentation at DDL 2023, 2023.
- Antoniak D et al, “Inhaled biologics: from nebules to SMIs – Dornase alfa in MRX004”. Poster Presentation at DDL 2023, 2023.
- “Home Nebulizer”. Cleveland Clinic, Web Page, Accessed Mar 2024.
- Kamin W et al, “A Handling Study to Assess Use of the Respimat® Soft Mist™ Inhaler in Children Under 5 Years Old”. J Aerosol Med Pulm Drug Deliv, 2015, Vol 28(5), pp 372–381.
- Watchel H, Jensen B, Kamin W, “Soft Mist Inhalers, from which age can they be used and what are the limitations?”. Presentation at DDL 2017, 2017.
- Woodcock A et al, “The environmental impact of inhaled therapy: making informed treatment choices”. Eur Respir J, 2022, Vol 60(1), Article 2102106.
- Goulet B, Olson L, Mayer BK, “A Comparative Life Cycle Assessment between a Metered Dose Inhaler and Electric Nebulizer”. Sustainability, 2017, Vol 9(10), Article 1725.
- “Cost of living crisis: 1 in 5 people with asthma surveyed say price hikes causing asthma attacks”. Asthma + Lung UK, Sep 2022.
- Ortsäter G et al, “Incorporating the Environmental Impact into a Budget Impact Analysis: The Example of Adopting RESPIMAT® Re-usable Inhaler”. Appl Health Econ Health Policy, 2020, Vol 18(3), pp 433–442.
- Dal Negro RW, Povero M, “Acceptability and preference of three inhalation devices assessed by the Handling Questionnaire in asthma and COPD patients”. Multidiscip Respir Med, 2016, Vol 11, Article 7.
- Kendrick AH, Smith EC, Wilson RS, “Selecting and using nebuliser equipment”. Thorax, 1997, Vol 52(Suppl 2), pp S92–S101.
- Dal Negro RW, Povero M, “The economic impact of educational training assessed by the Handling Questionnaire with three inhalation devices in asthma and Chronic Obstructive Pulmonary Disease patients”. Clinicoecon Outcomes Res, 2016, Vol 8, pp 171–176.
- Vizzoni L et al, “Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment”. Pharmaceutics, 2023, Vol 15(6), Article 1618.
Previous article
ADDRESSING CNS THERAPIES THROUGH NOSE-TO-BRAIN PATHWAYSNext article
SCALING GLP-1 DRUGS WITHOUT A MOUNTAIN OF WASTE
