Citation: Flodin A, “Spray Characterisation Testing”. ONdrugDelivery, Online, April 20, 2021.
Ann Flodin introduces the company’s SprayVIEW® measurement and automated actuation stations for the development and quality control of container closures and finished products.
SPRAYVIEW® MEASURING SYSTEMS BY PROVERIS
Recipharm has the expertise and specialised equipment to support the development of quality analytical techniques for the development of inhaled products, specifically nasal and oral sprays, and pressurised metered-dose inhalers (pMDIs). This includes the company’s SprayVIEW® measurement and automated actuation stations, developed by Recipharm’s expert development team. SprayVIEW® is a registered trademark of Proveris Scientific.
Inhalation products are extremely complex to develop and manufacture, and it is important to understand potential interactions between the formulation and the delivery device throughout the development stages.
Spray pattern and plume geometry are critical attributes when characterising nasal and oral sprays and pMDI aerosols, and are critical for conducting accurate and consistent spray pattern and plume geometry tests (Figure 1).
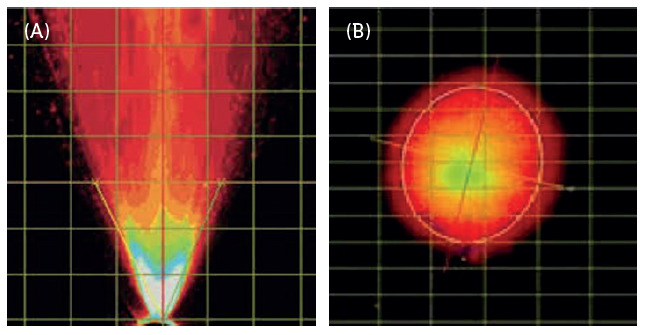
Figure 1: (A) Plume geometry and (B) spray pattern.
The acquisition of real-time images of aerosols or sprays emitted from a device, using a high-speed camera, enables subsequent analysis of the spray pattern and plume geometry, which represents the shape of the aerosol or spray. Recipharm’s automated MDx and NSx actuation stations allow for consistent actuation using parameters such as actuation velocity and acceleration, minimising intra- and inter-device variability. The NSx and MDx are automated test stations for actuating nasal spray products and pMDIs, respectively.
Actuation graphs, derived from data collected during spray pattern and plume geometry measurements, display the actuation profile to determine the force-to-actuate profile of the device.
SprayVIEW® measurement systems are not only valuable tools to guide drug product development, but can also be used to support quality control for container closure components (i.e. valves, pumps) and finished drug products.
As part of Recipharm’s spray pattern and plume geometry test service, the company offers inhalation and nasal spray product development from initial formulation and process development to registration, commercial batch release and product characterisation. Recipharm carries out in vitro bioequivalence (IVBE) studies in generic inhalation and nasal spray product development, and uses its expertise to develop and validate SprayVIEW®-based methods for aerosol and nasal spray testing. The company also provides support with device-related changes during the product lifecycle (Figure 2).

Figure 2: Recipharm offers inhalation and nasal spray product development.
WHY CHOOSE RECIPHARM INHALATION SOLUTIONS
Recipharm Inhalation Solutions™ provides a comprehensive, end-to-end service, which manages complexity and reduces risk for its customers. With its experience and track record, Recipharm is a leading CDMO in the inhalation field, with a long history of inhalation drug product and device development and manufacturing. Recipharm’s depth of knowledge enables it to overcome the challenges associated with inhalation drug products and devices; by developing inhalation products with the device and commercial manufacture in mind, the company eliminates hurdles and reduces the time to market.
Recipharm has facilities in the US and EU dedicated to inhalation development and manufacturing, including a large pilot plant for clinical manufacture and small-scale manufacture. In addition, its services are supported by strong corporate health, safety, environment and cGMP quality systems to ensure the highest levels of compliance across worldwide markets.
Previous article
HOSTAFORM® POM ECO-B – PROVEN, VERSATILE, EASY AND SUSTAINABLENext article
LINKING THE “D” & “M” IN CDMO
