Citation: Goldfarb L, “Streamlining the Testing Process for Autoinjector Devices”. ONdrugDelivery, Issue 166 (Oct 2024), pp 34–38.
Landon Goldfarb highlights the challenges of autoinjector testing and the importance of drawing on the experience of customers when it comes to drug delivery device innovation.
The injectable drug landscape has shifted significantly in the last 10 years, with autoinjectors quickly becoming the preferred choice for chronic conditions and, more interestingly, an expected option for many patients. The pace of innovation within the biologics and other large-molecule formulation space has, in many ways, prompted this shift from the pharmaceutical side, as existing technologies, such as prefilled syringes, became less equipped for safe, efficient delivery to the patient.
The larger volumes and higher viscosities of these formulations created more opportunities for patient misuse and made manual delivery more difficult for certain segments of the patient population. These technical hurdles catalysed the interest in autoinjectors, which allow for more reliable and repeatable drug delivery with minimal opportunity for error. When coupled with a growing infrastructure to support large-scale fill-finish and assembly, autoinjectors represented a massive opportunity for pharmaceutical manufacturers to better differentiate their products and appeal directly to healthcare professionals and patients.
A major concern for healthcare providers is ensuring that patients adhere to their prescribed dosing regimen in order to receive the full therapeutic efficacy of the drug. Several studies have characterised the positive effects that self-administration devices have had on overall patient compliance. In addition, the use of self-administering devices provides more opportunity for integrating connectivity capabilities, offering the physician reliable data to confirm the treatment is being followed correctly.
“The design of autoinjectors offers significantly more opportunities for customisation to better align the device with the indication and patient population.”
From a patient’s perspective, the overall usability and experience are improved using autoinjectors, especially for the population segment that is afraid or anxious around needles. The design of autoinjectors also offers significantly more opportunities for customisation to better align the device with the indication and patient population. For example, autoinjectors used for patients with severe forms of arthritis will be ergonomically designed to minimise pain during use. These features all work to increase patient confidence in their device and ultimately improve the patient experience.
MECHANICAL TESTING IMPLICATIONS
All these improvements for patients and healthcare professionals represent a major shift in the complexity and variability of drug delivery devices and, as such, impact their requirements for mechanical testing. In a departure from more traditional devices, such as syringes and cartridges, autoinjectors use automated functions, such as passive safety and activation mechanisms, with auditory and visual feedback to alert the patient to the beginning and completion of the injection.
This functionality is far beyond the standard scope of mechanical test systems, requiring measurements aside from force and displacement to assess the performance of the injection and the patient feedback. These requirements have been described within the ISO 11608-5 standard and, more recently, within the draft guidance from the US FDA regarding Essential Drug Delivery Outputs (EDDOs) and can include the following evaluations:
- Dose accuracy
- Needle depth
- Cap removal force
- Activation force
- Injection time
- Audio/visual tactile feedback.
“Device specimens can often be at a premium, especially early in the development process, and their design prevents them being retested in most cases.”
In many labs, the standard practice is to evaluate these characteristics by performing individual tests on separate pieces of equipment. In addition to the time and effort required to do this testing, there are other significant drawbacks to this approach. Device specimens can often be at a premium, especially early in the development process, and their design prevents them being retested in most cases. For this reason, project timelines and cost can be strained when large numbers of specimens are needed for testing.
The variety of form factors and patient-device interactions also complicates the scope for performing full functionality in a single test run. Figure 1 shows the myriad devices that exist on the market today, making a one-size-fits-all solution difficult. Furthermore, the ISO standard provides minimal direction for test set-up or procedure, opting instead to be purposefully broad to promote innovation in autoinjector design. This open-endedness means that testing procedures vary widely across device manufacturers and pharmaceutical companies.

Figure 1: Mechanical testing system with a range of drug delivery devices.
The challenges outlined here highlight the need for a comprehensive functional tester for autoinjectors with proven methodologies for measuring the parameters, the flexibility to work with a range of devices and the necessary automation to perform the testing in a single sequence. Integration of the necessary hardware to perform this testing on a traditional mechanical testing system is only part of the challenge – designing a user experience to allow for simple development of methods, modification of testing parameters and process control is equally critical.
“Instron’s mechanical testing systems have been around for over 75 years and are trusted to validate the safety and performance of materials and products on a global stage.”
AUTOINJECTOR FUNCTIONALITY TEST SYSTEM FOR EDDO ANALYSIS
Instron’s mechanical testing systems have been around for over 75 years and are trusted to validate the safety and performance of materials and products on a global stage. Core to Instron’s beliefs is the importance of customer-backed innovation – building on the experience of customers to develop products that meet their needs and address the current challenges of the market.
The exponential growth of the autoinjector market has served as a catalyst for Instron to collaborate with industry partners to develop a solution for testing the evolving autoinjector. Creating the complete set of requirements for this system necessitated understanding the pain points across the entire drug development process, as well as understanding the unique needs of device manufacturers, pharmaceutical companies and contract development and manufacturing organisations (CDMOs) in both R&D and production environments. Many of the insights gained dealt with two main topics –system flexibility to support different device types and system usability.
SYSTEM FLEXIBILITY
The autoinjectors on the market have distinct geometry and functionality to support their specific patient populations. This variability necessitates thoughtful design to accommodate the variation of device interfaces, injection mechanisms, primary packaging, patient feedback and failure modes. Figure 2 highlights just a few of the different device variables and their impact on the system, as well as measured results.
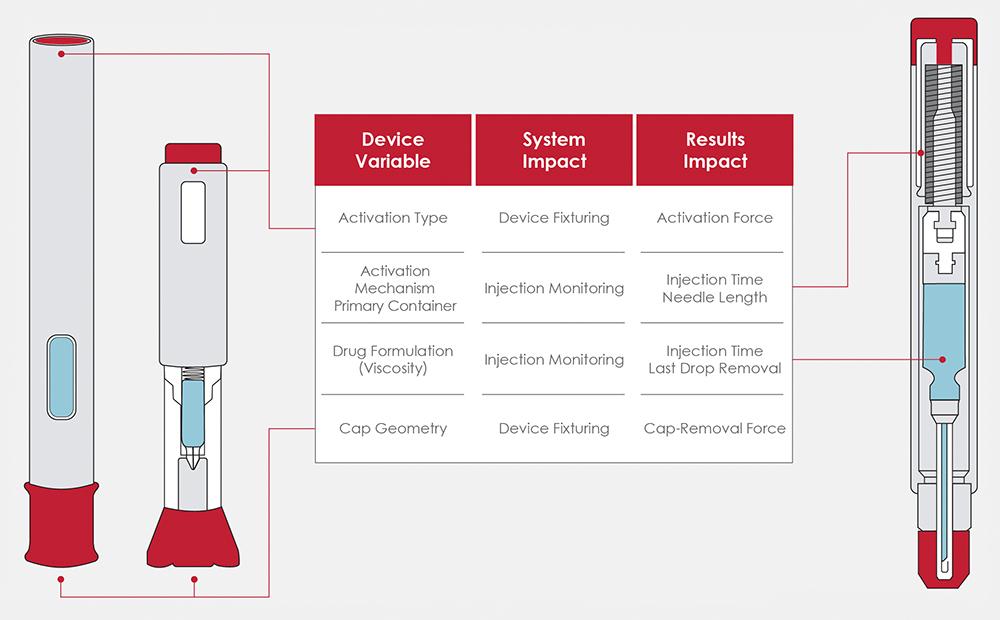
Figure 2: Overview of device variables and testing impacts.
Cap removal is often the first test performed and can be overlooked as a straightforward evaluation. Internal testing and customer feedback highlighted the correlation between device alignment and the repeatability of the cap-removal force. Data showed that any device misalignment could artificially raise the measured force by up to 20%. Additionally, minimising external influence is also preferred, so simple tooling was developed to match the cap geometry of common devices and easily interface with the system, as seen in Figure 3. The tooling design purposefully allows for customer-driven design in instances where device manufacturers are developing their own custom devices or customising stock devices to their specific needs.
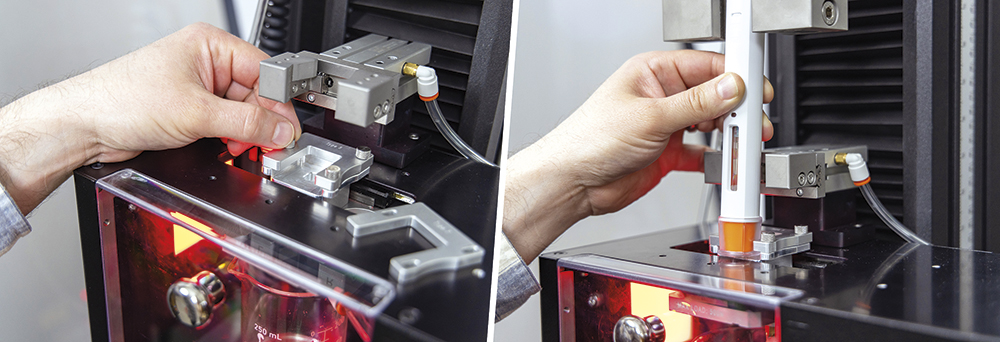
Figure 3: Easily interchangeable inserts designed to fit commercially available devices.
The primary container and drug formulation also have significant impacts on the device functionality and, subsequently, the required system capability. The primary container for most fixed-dose devices is a prefilled syringe, and its properties will rely on the formulation and delivery route. One of the EDDOs – needle length – is related to the pharmacokinetic properties of the formulation, which will often dictate the required delivery route.
“The testing system uses a machine vision camera designed to identify the needle during the injection and then monitor the tip position throughout the injection.”
For example, the delivery route for parenteral injection will be via either the subcutaneous or intramuscular regions, where the needle depth can range from 3–20+ mm, depending on the target delivery region. To compensate, the testing system uses a machine vision camera designed to identify the needle during the injection and then monitor the tip position throughout the injection, providing the needle depth during injection. To provide additional value and better assess the device performance, the camera will simultaneously monitor the drug delivery and capture the length of the needle at the start of the injection to better evaluate the delivery region over time. This additional analysis can be used as an attribute analysis, confirming whether the complete injection was delivered within the therapeutically relevant region.
Viscosity has been another major topic within the industry, as the advent of new drug classes, such as biologics and other large-molecule formulations, has led to viscosities that are orders of magnitude greater than traditional formulations. Fluid viscosity is of primary importance during the device selection process, as increased viscosities require activation mechanisms or powerpacks that can sufficiently deliver the drug within an acceptable duration – typically less than seconds. The force profile of the powerpack will also need to be finely tuned to ensure that excessive force is not generated at activation, which can cause shearing of the prefilled syringe flange. All of this contributes to variations in injection time and needle velocity.
Using the machine vision camera and tracking the fluid directly provides an extremely robust methodology for determining the injection time that is less affected by these variables. Alternative methodologies, as suggested in ISO 11608-5, such as laser- or gravimetric-based systems, each have drawbacks. Laser-based systems are direct but susceptible to external variables such as changing lighting conditions and mist produced from the injection. Gravimetric systems are indirect and require complex algorithms to adequately identify the start and end of injection for a range of mass profiles, which are highly dependent on formulation viscosity.
In many cases, companies may be testing safety syringes and prefilled syringes in addition to autoinjectors. Instron’s Autoinjector Testing System is designed to accommodate these devices as well as perform EDDO evaluations. Using either Instron-provided or customer-designed inserts, the system can be adapted to evaluate properties such as rigid needle shield removal, break-loose and glide force and plunger lockout checks.
SYSTEM USABILITY
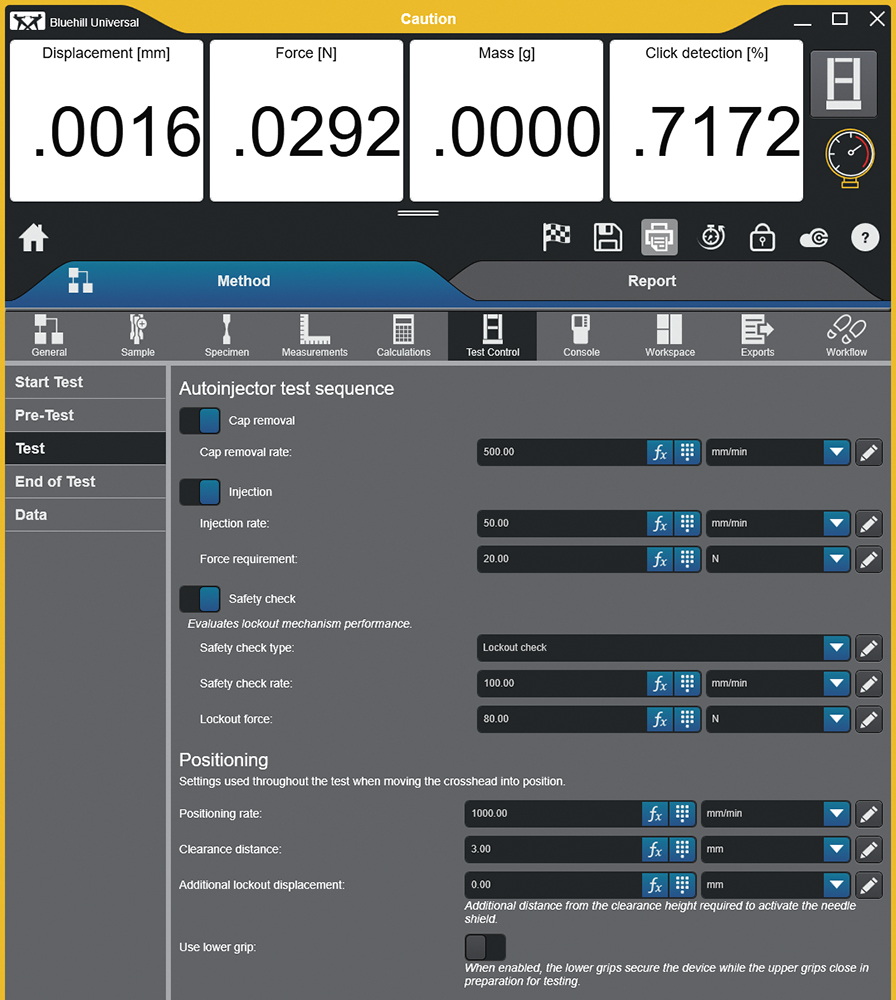
Figure 4: Test set-up with operator-defined critical parameters in Bluehill Universal software.
Where system flexibility is all about what the system is capable of, system usability is about how an operator interacts with the system. These systems are essentially fully automated, performing the tests sequentially without manual intervention. Accomplishing this level of automation – while simplifying the user interface and enabling independence for users to develop their own methods – was a foundational requirement of the product. Although R&D and CDMO environments are entirely different, they share a requirement for ownership of method development.
In an R&D environment, a level of experimentation is performed, especially when characterising a device, requiring the ability to easily modify test methods. For a CDMO, agility is paramount – client-driven method changes or the addition of new client projects must be acted on quickly, without requiring vendor support. Figure 4 shows an example of the test method development screen, designed from the bottom up to perform autoinjector testing and using customer feedback to isolate the critical parameters, which need to be user definable, while hiding everything else.
“The system is designed to provide qualitative insights into the testing to aid in root cause analysis.”
Production testing is under intense scrutiny and any failures need to be noted and addressed through root cause analysis. Given the implications for even small delays in resolving, the system is designed to provide qualitative insights into the testing to aid in root cause analysis. High-definition video of the injection area is recorded and saved with the samples to help identify any visual clues for issues such as an occluded needle or excessive needle angle. Additionally, the video is correlated with the graphical display, allowing the operator to select a point of interest on the curve and find the corresponding video frame. Timestamped images of the needle at both the start and end of the injection are saved as separate files to confirm the reported needle depth values are representative of the correct needle position.
In GMP environments, system suitability is a daily requirement and, in many labs, the process is primarily paper driven, with sign-offs being performed in lab notebooks and no control over whether the daily check is performed. To alleviate these issues, the system software will automatically integrate the daily check procedure, locking out a user from testing until they have performed the necessary tests. The data and report are stored in the audit log, stamped with the date, time and operator associated with those tests.
Figure 5 shows an operator performing a system suitability test for the machine vision camera. Using Instron’s TrendTracker module, long-term data analysis of the transducer performance can be checked, providing evidence of degradation of the transducer performance and helping to catch an issue before it affects data. This system is designed to digitise the entire system suitability process and increase transparency, giving lab managers assurance that the test systems are being used properly.

Figure 5: Operator uses gauge pins to verify camera performance for system suitability testing.
Instron’s next-generation autoinjector is the product of years of working with industry partners, distilling the core requirements for evaluating these devices and understanding the features necessary to optimise usability. Additionally, the ability to test thousands of different devices with a wide range of form factors and formulation variables enabled Instron to quickly find common pitfalls and helped to develop more robust solutions for this market. This system is designed to provide an industry-leading level of capability – flexible enough to support R&D work while robust enough to withstand the rigor of production – all in a package dedicated to enhancing the user experience.

