To Issue 159
Citation: Osorio S, Mou S, Dean C, “Sustainable by Design: Developing Patient- and Planet-Centric Medical Devices”. ONdrugDelivery, Issue 159 (Apr/May 2024), pp 52–57.
Su Osorio, Stephanie Mou and Charlie Dean, explore the delicate balance between patient and planet centricity and propose a user- and planet-centric approach for the development of sustainable medical devices.
The landscape of medical devices is rapidly evolving, driven by a dual focus on patient centricity and sustainability. Ensuring the usability, safety and adaptability of medical devices to real-world use scenarios is a must. At the same time, it is becoming increasingly urgent to consider the environmental impact of the device throughout its full lifecycle. The aim is to maximise benefit to patients today, while considering the future.
This article explores the delicate balance between patient and planet centricity. It proposes a user- and planet-centric approach to developing valuable and sustainable medical devices by marrying user-centred design and sustainability best practices, applied by a multidisciplinary team. This blended methodology supports better decision making rooted in user insight and informed by environmental impact. It generates market differentiation by offering concrete added value for the users with seamless sustainability.
“Responsible pharmaceutical companies must consider not only the patient outcomes but also the environmental impact of their medical devices.”
THE CASE FOR PATIENT-CENTRIC AND PLANET-CENTRIC MEDICAL DEVICES
Patient centricity places the user at the heart of solution design.1 The goal is to create medical solutions that are adopted, used consistently and correctly, and deliver the intended patient outcomes. To achieve this, design decisions are centred around user experiences. With the push for patient-friendly and effective self-care medical solutions for home use, the significance of patient centricity continues to grow.
The path to a healthier and greener future involves advancement in both patient centricity and environmental stewardship. Responsible pharmaceutical companies must consider not only the patient outcomes but also the environmental impact of their medical devices. As pharma embraces the challenge of balancing patient and planet centricity, this article outlines an approach to the development of inherently user-focused and sustainable medical devices.
A PLANET-CENTRIC APPROACH DRIVEN BY USER VALUE
Organisations often struggle with the challenge of designing sustainable products without compromising user value and outcomes. They launch products that fail to deliver added value compared with alternatives, assuming sustainability will stand as a differentiator. The adoption of these products is limited, and users usually revert to alternatives following disappointing experiences with “sustainable” options.
In healthcare, the gap between user outcomes and environmental impact is even more pronounced. The value of a medical product lies in the clinical outcome it delivers. Sustainability is a desirable attribute rather than a differentiator. This poses the question: “How can we address sustainability in an impactful way while maintaining a valuable medical solution, thereby increasing the likelihood of adoption compared with alternatives in the market?”
This article illustrates an approach to the patient- and planet-centric design of medical devices, applied to a conceptual body-worn insulin injector.2 Drug-device combination products play a pivotal role in healthcare by administering consistent and precise medication dosing, which empowers patients requiring regular injections to carry on with daily activities. User centricity is particularly relevant for devices such as these, typically handled by patients and their caregivers.
In the following sections, the descriptor “user” over “patient” is adopted. To understand the people we design for, we must recognise that, beyond patients, they are multifaceted individuals with needs, emotions, values and attitudes. Moreover, other users besides patients must also be considered, such as caregivers and healthcare providers.
THE APPROACH
A user-centric and planet-centric approach requires a multidisciplinary team. There is a need for a deep understanding of the users, the medical device, sustainability and their intricate dynamics. This demands appropriate data inputs and a skilled team for the analysis. Only a multidisciplinary team will be well equipped to, together, navigate the complexity of competing requirements and trade-off decisions arising from balancing user needs and sustainability considerations.
In this approach, a multidisciplinary team typically comprises sustainable medical technology engineers, human factors engineers, industrial designers and design strategists. Each discipline represents a perspective and leads a workstream. Together, they review existing medical devices, align on opportunities for redesign and manage trade-offs to reach better patient and planet outcomes in a future device that they co-create.
THE DATA
This approach calls for two research questions – one focused on the user, the other on the planet. Human factors engineers explore the question: “How is the user experience of the medical device?” They perform a task analysis using the PCA (perception, cognition, action) framework and investigate common usage errors through a review of publicly available resources, such as the MAUDE database,3 scientific literature and patient forums, gathering insight into the user, their experience and usability.
Sustainable medical technology engineers investigate the question: “What are the environmental impacts at each step of the user journey?” They conduct a lifecycle assessment (LCA) to identify areas that present the most significant opportunities from a sustainability standpoint.2 By identifying the materials, weights, manufacturing, distribution and disposal processes for a generic body-worn insulin injector and co-products, its impact can then be calculated with the SimaPro (Amersfoort, the Netherlands) LCA tool, using averaged impact data from the Ecoinvent (Zurich, Switzerland) database.
“Opportunities for redesign exist where there is the greatest potential for improvement in both the user experience and the sustainability impact of the device.”
THE USER JOURNEY MAP METHOD
A user journey map (Figure 1) is a helpful way to synthesise knowledge of the user experience and the sustainability impact of the device. This is a user-centred design method that structures synthesised research information to facilitate its analysis within the context of the user experience.4 It outlines the key stages and activities of user interaction with the device. Underneath, it maps the “user emotional journey”, and displays the peaks and troughs of the “user gains” and “pain points”. An “ideal journey” shows everything proceeding as expected, whereas a “real-life” journey reflects prevalent issues. The shaded region between them displays the spectrum of user experiences in between. The “sustainability journey” is then overlaid, its peaks and troughs mapping the relative sustainability impact at each “real-life” journey step.
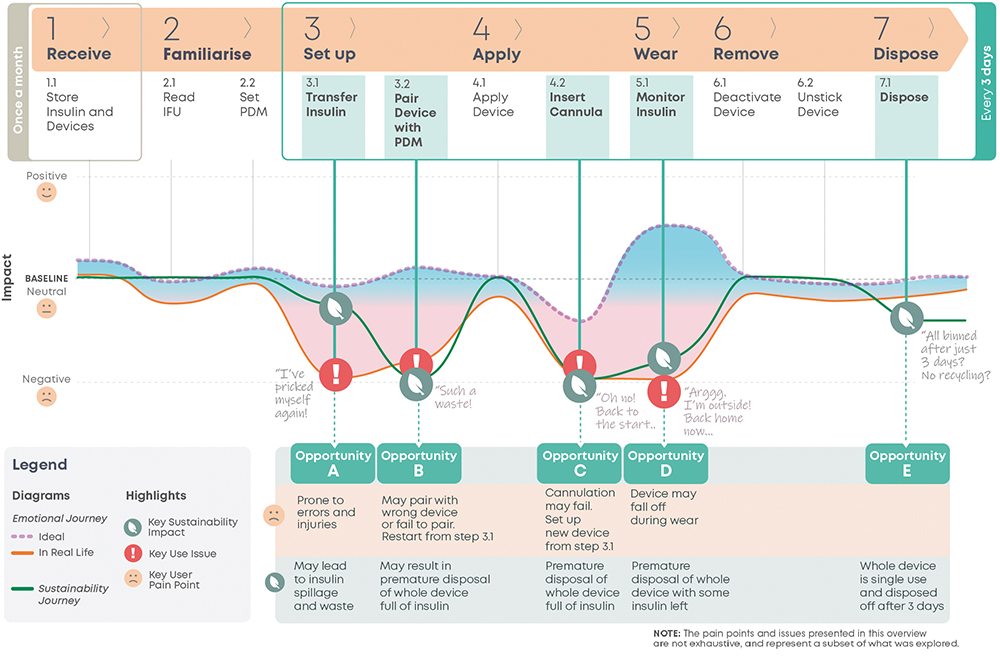
Figure 1: Current user journey map.
Opportunities for redesign exist where there is the greatest potential for improvement in both the user experience and the sustainability impact of the device. The current user journey map details the user experience and the relative sustainability impact of the current device over its use steps, which enables the identification of these overlapping areas of potential. This analysis has highlighted five key opportunity areas.
Opportunity A – Transfer Insulin
Insulin transfer is prone to user errors and injuries, spillage and wastage. While insulin wastage was found to be negligible from the LCA using pharmaceutical company data, it is a strong “pain point”, as highlighted by the significant dip in the emotional journey at this step (Figure 1, Step 3.1).
Opportunity B – Pair device with PDM
A common issue is the integrated personalised diabetes management (PDM) pairing with the wrong device or failing to pair (Figure 1, Step 3.2). If pairing cannot be completed, the user disposes of the device full of insulin and restarts set-up with a new device from Step 3.1, Transfer Insulin. This is time consuming and upsetting for the user. The premature disposal of the device also has a negative environmental impact.
Opportunity C – Insert Canula
Cannula insertion failure is the most common issue in the MAUDE database (Figure 1, Step 4.2). It requires setting up a new device from Step 3.1 Transfer Insulin. This will be quite frustrating for the user, who will need to restart again after being further along in their device application journey. From a sustainability point of view, Opportunity C is of equivalent impact to Opportunity B.
Opportunity D – Monitor Insulin
The device falling off while being worn (Figure 1, Step 5.1) is the second most common issue in the MAUDE database. The user must then dispose of the device and set up a new one. This often occurs during activity, in settings where it is less convenient to set and apply a new device. Opportunity D is of equivalent environmental impact to Opportunity B and C, or slightly better if the fault happens closer to the end of life of the device.
Opportunity E – Dispose
Every three days, the device reaches its end of life and is disposed of. While not a particularly painful pain point for users, more for the environment, patient forums present enquires from users about how to extend the lifetime of their devices. Those enquiries highlighted this as another opportunity for redesign.
Opportunities B, C and D show high potential to improve both user experience and device sustainability and are thus prioritised. Furthermore, users are creating and sharing workarounds for reusing insulin from failed devices. They seem to perceive the most value to be in the insulin itself and the device just as a therapy enabler. While insulin waste has negligible sustainability impact, these workarounds signpost a strong pain point. Thus, the possibility of safely and easily reusing insulin from failed devices should also be explored.
“The PCBA has an especially high environmental impact. By incorporating it in a reusable module, its impact is amortised and reduced considerably.”
CO-CREATING IMPACTFUL SOLUTIONS
A proposed future device is to be developed around the key opportunities for redesign. First, the current device is decomposed into high-level, solution-agnostic functions. Then, functions are categorised according to environmental damage, failure rate, cost, sterility and time consumption. Finally, functions are allocated to logical subassemblies to minimise Pain Points and environmental damage (Figure 2).
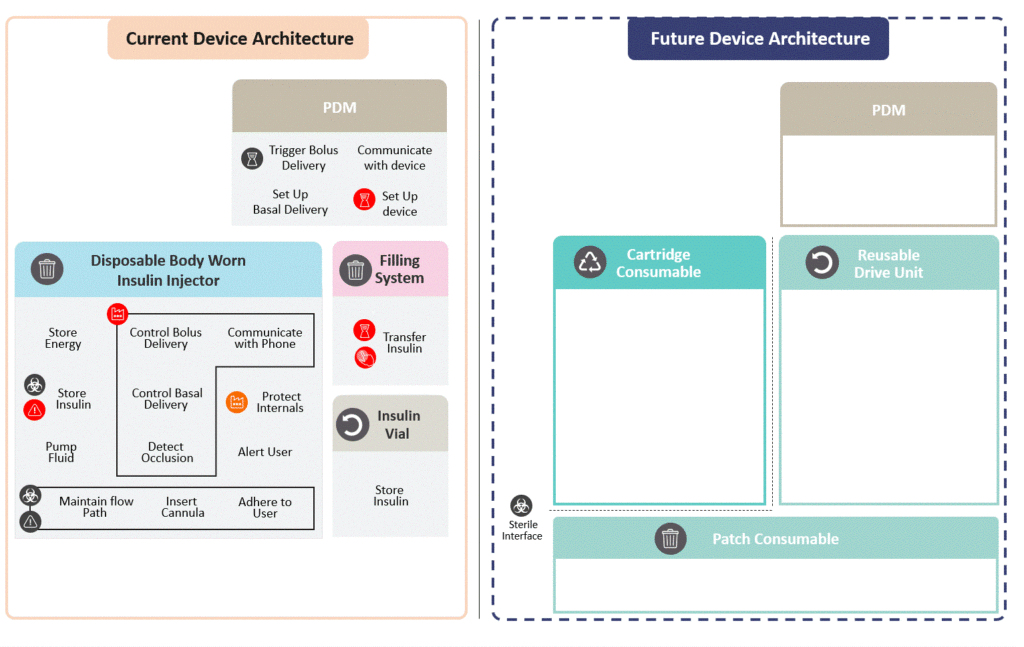
Figure 2: Device architecture redesign.
The proposed future device (Figure 3) comprises three modules, assembled by the user to form a fully functional device. One module is reusable 10 times over a month, and two are consumables replaced every three days.
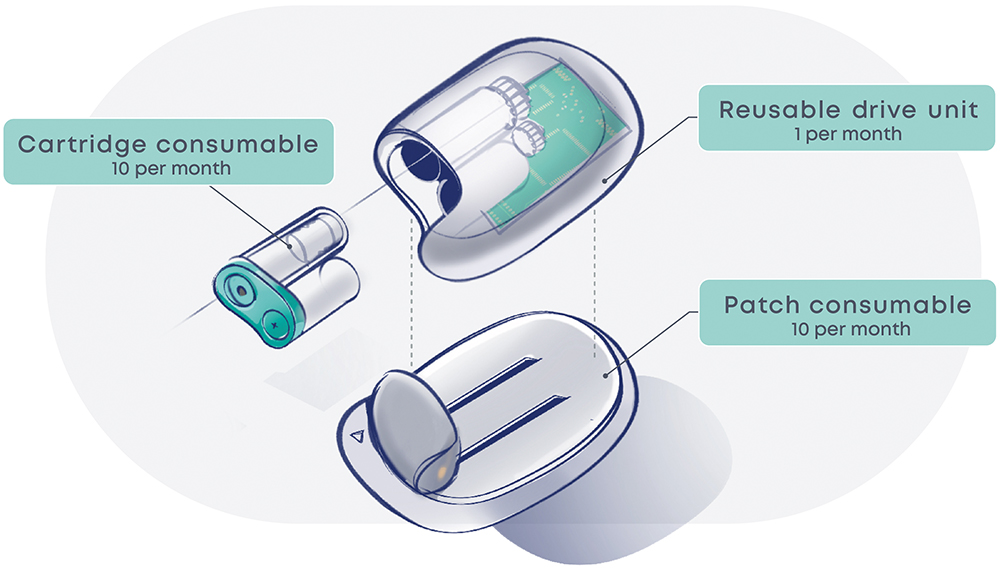
Figure 3: Proposed future device.
The Reusable Drive Unit contains the printed circuit board assembly (PCBA) as well as the insulin pump. The PCBA has an especially high environmental impact. By incorporating it in a reusable module, its impact is amortised and reduced considerably. Additionally, this reduces the pain point of pairing a new pump from occurring once every three days to once a month.
The cartridge consumable contains the prefilled insulin vial and batteries. The pain point of transferring insulin is eliminated by replacing this step with a simple clipping operation. Consequently, failures in other modules will not result in insulin wastage. Disposable batteries are opted for, as the LCA identified that batteries have a relatively low environmental impact, and this avoids additional charging infrastructure and user burden.
The patch consumable sticks to the patient and contains the cannulation and cartridge-piercing systems. These are the most common sources of failures. With a separate architecture, a failing patch can be replaced and a premature disposal of the whole device prevented.
“To enable a modular device, some trade-offs need to be considered, with ongoing evaluation and refinement as the design detailing progresses.”
EVALUATING THE SOLUTION
To assess how the future device concept compares with the current device on user experience and sustainability impact, a future user journey map (Figure 4) is used. It projects the future user experience and sustainability impact of the proposed future device. By contrasting this with the current user journey map, the impact on the five key opportunity areas can be assessed.
Opportunity A – Insulin Transfer Challenges
A cartridge module prefilled with insulin that will be loaded into the drive unit (Figure 4, Step 3.2) eliminates the original pain point.
Opportunity B – Pairing Issues
Pairing is now only required once a month (Figure 4, Step 3.1), reducing both the pain point frequency and the potential number of devices prematurely disposed of due to pairing issues. Additionally, when pairing fails, only the drive unit needs replacing, as opposed to the whole device in the original solution.
Opportunity C & D – Cannulation Failures and Device Falling Off
In both cases, now only the patch module needs to be replaced (Figure 4, Steps 4.3, 5.1), reducing the sustainability impact of these failures. The user will now restart from “Apply Patch” (Figure 4, Step 4.1), reducing the time impact and task burden of this failure on the user, when compared with restarting from “Transfer Insulin” in the original device.
Opportunity E – Device Disposal
The drive unit houses the components with the highest environmental burden and is now used for a month (Figure 4, Step 7.2). This single change effectively reduces impact across a range of categories, such as global warming, resource usage and damage to ecosystems, by up to 50%.
Overall User Impact Considerations
To enable a modular device, some trade-offs need to be considered, with ongoing evaluation and refinement as the design detailing progresses. Overall, the changes described reduced the total time, effort and task burden of the user. The fiddly insulin transfer is replaced with clipping a cartridge. The failure-prone drive unit pairing and deactivation steps are now only occurring once a month. To enable these changes, three simple clipping and unclipping steps are added to apply and remove the device (Figure 4, Steps 4.2, 6.1, 6.2). Due to the technical constraints of the modular system, cannulation will likely be manual, which may create more discomfort than the current automatic insertion. Other aspects for further evaluation in the detailed design include the impact on device size, the potential effect of module interface exposure to the environment and ways to reduce the potential waste of prefilled cartridges by patients with low insulin requirements.
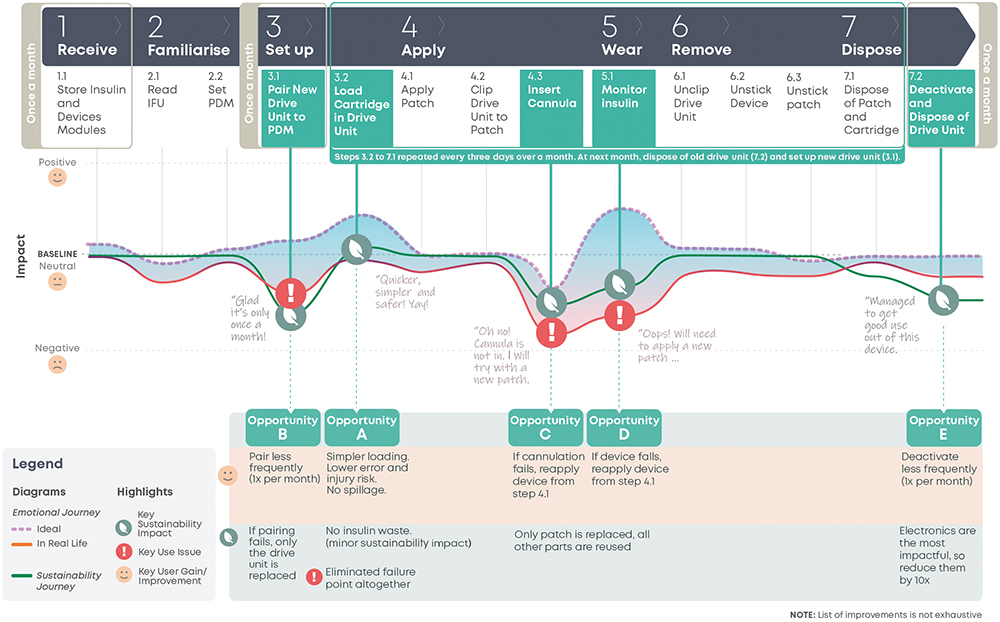
Figure 4: Future user journey map.
CONCLUSION
A user- and planet-centric approach to medical device development encompasses a comprehensive review of the current medical device, followed by the identification of opportunity areas for impactful redesign. This process leads to the generation of innovative design concepts for a future device, with a dual focus on enhancing patient value and minimising environmental impact. These are then integrated with a thorough evaluation of trade-offs, ensuring a balanced approach to the device development.
The successful implementation of a user-centric and planet-centric approach relies on the collaboration of a multidisciplinary team, guided by three key principles:
- Empathise deeply: Understand user needs and experience and identify where value can be added.1
- Leverage insights: Prioritise solving user experience aspects that are simultaneously the biggest pain points and most environmentally damaging.2
- Embed sustainability: While addressing the biggest pain points, strive for “invisible sustainability”, removing the burden of trade-off decisions from the user.
This approach provides a roadmap for balancing the needs of people and the planet. It guides pharma organisations through medical product development with empathy for their users and responsibility towards the planet. It delivers devices that stand out by providing a superior user experience and are inherently sustainable. These devices will be well positioned to become the preferred choice and, through them, leading pharma companies are assuming their part in fostering the adoption of more sustainable devices.
ACKNOWLEDGEMENTS
Paramesh Natarajan – Systems and Design Control, Cambridge Consultants Pierre-Francois Gautier – Human Factors Engineering, Cambridge Consultants Viviane Mosso – Medical Technology Design, Cambridge Consultants
REFERENCES
- Algorri M et al, “Patient-Centric Product Development: A Summary of Select Regulatory CMC and Device Considerations”. J Pharm Sci, 2023, Vol 112, pp 922–936.
- Natarajan P, Bavar S, Dean C, “How Lifecycle Assessment Supports Insight-Driven Sustainable Design”. ONdrugDelivery, Issue 153 (Oct/Nov 2023), pp 16–19.
- “MAUDE – Manufacturer and User Facility Device Experience”. US FDA, accessed Apr 2024.
- Roesch N, Tiberius V, Kraus S, “Design thinking for innovation: context factors, process, and outcomes”. Eur J Innov Manag, 2023, Vol 26(7), pp 160–176.

