To Issue 149
Citation: Fechant C, Hilliquin L, “Taking a Patient-Centric Approach to Cancer Care Through Immunotherapy.” ONdrugDelivery, Issue 149 (Jun 2023), pp 33–36.
Corinne Fechant and Laura Hilliquin highlight the role digital health solutions can play in supporting patient self-administration for the increasing number of immunotherapies for cancer treatment.
“Immunotherapies can not only enable the immune system to remember what cancer cells look like but also adapt to changes in cancer cells.”
Immunotherapy has transformed cancer care with immune checkpoint inhibitors, providing efficient stimulating agents that act on patients’ own immune systems. The response to these treatments drives long-lived tumour destruction with significant benefits for patients who have made minimal progress, or even relapsed, when using more traditional treatment methods. At the same time, these immunotherapies can present self-administration challenges and come with side effects that other therapies do not have, which can make self-management of care difficult.
Overcoming these two obstacles requires a patient-centric approach that is enhanced by the use of digital technologies that work alongside immunotherapy. By helping patients self-manage their treatment through education and guided decision making, digital products can improve the effectiveness of immuno-oncology, which can lead to better clinical outcomes and quality of life for patients.
THE BENEFITS OF IMMUNO-ONCOLOGY
The use of immunotherapies in cancer treatment is meant to help use the body’s immune system to prevent, or even eliminate, cancer. Essentially, immunotherapies enable the immune system to recognise the difference between healthy cells and cancerous cells.
Patients with cancer benefit from immuno-oncology largely because treatments are targeted to the specific white blood cells (lymphocytes) that evoke an anti-tumour immune reaction. It is common for immunotherapies to be prescribed in combination with other treatments, such as surgery, radiation, chemotherapy and other targeted therapies.
Highly targeted therapies are proven to be more effective at treating certain types of cancer, with a reduced likelihood of relapse compared with traditional treatments, such as radiation or chemotherapy, on their own. Additionally, immunotherapies can not only enable the immune system to remember what cancer cells look like but also adapt to changes in cancer cells, which further contributes to the long-lasting effectiveness of immuno-oncology.1–3
“Patients may need extensive training on self-administration to make sure that injections are done correctly and that they feel confident enough.”
UNDERSTANDING THE PATIENT JOURNEY
Following an initial biopsy to confirm a cancer diagnosis, it is common for patients to be prescribed a generalised treatment to shrink a tumour prior to surgery or to avoid the risk of recurrence after surgery. Also, some patients fail to respond to traditional treatments. Others may see their symptoms persist or may suffer a relapse after an initial treatment appears to have succeeded. In these cases, an oncology care team may order an additional biopsy to determine if a patient has a biomarker for a particular antibody that could be targeted by targeted therapy or immunotherapy. If a biomarker such as PDL1 is present – or not, in some cases, as it is not required in some indications – then immunotherapy can be prescribed, and a new course of treatment can begin.
As immunotherapies continue to be brought to trial, approved and used more and more in real-world settings, and as access to genetic testing improves, it is hoped that these therapies will become more readily and widely available to patients for the early stages of treatment. This could help more patients receive the right treatment at the right time – improving both clinical outcomes and overall quality of life, while potentially contributing to lower care costs downstream.
SPECIFIC CHALLENGES FOR IMMUNO-ONCOLOGY
While immuno-oncology presents many clear benefits to patients with cancer, as well as their care teams, there are two areas where immunotherapies – and especially immune-checkpoint inhibitors such as CTLA-4 – pose challenges when compared with more traditional treatment methods (Figure 1).
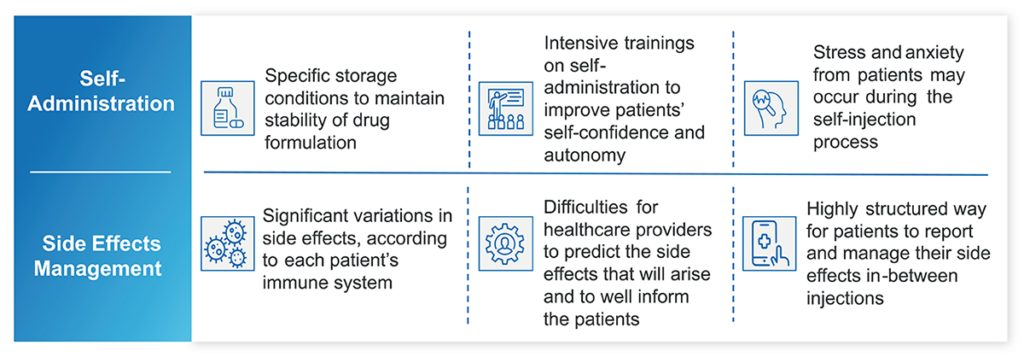
Figure 1: Real-world challenges for immuno-oncology treatments.
Administration
Self-administration has been one of the most powerful advances in managing chronic conditions – and cancer is no exception. Patients who can administer therapies from the comfort of their homes save themselves a trip to the hospital or oncology clinic and all the challenges that may present, from scheduling and transportation to time away from family or work.
The same is true for immuno-oncology, but the nature of these therapies requires additional consideration for patients and their care teams. Immunotherapies tend to be made up of larger molecules than other treatments and need to be well distributed throughout the body. When appropriate formulations are homologated and given these characteristics, immunotherapies such as immune checkpoint inhibitors will require subcutaneous administration, typically in the abdomen or thigh.
Self-administration devices must be developed accordingly, with appropriately sized needles and plungers. What’s more, maintaining the stability of the formulation often requires storage at a specific temperature or humidity, which could be difficult for certain people, given their living conditions. As a result, patients may need extensive training on self-administration to make sure that injections are done correctly and that they feel confident enough. Patients should also be taught how to store the medication, remove it from the refrigerator to achieve room temperature, aseptic administration technique, along with education on what to expect as they are injecting a therapy to achieve success. Care teams must be prepared to make special accommodations as necessary.
Side Effects
Traditional treatment options, such as radiation and chemotherapy, tend to have predictable side effects – notably nausea and hair loss. Surgical procedures also typically have predictable recovery timelines and expectations for regaining mobility or recovering appetite, for example.
This is not the case for immunotherapies. Because the therapy reacts to the patient’s immune system, side effects can vary significantly from one patient to another, depending on how their individual immune system responds. In fighting one type of cancerous cell, a therapy may activate the immune system and affect almost all tissues and organs with autoimmune disorders. For example, colitis was a common4 – and unexpected – side effect of some of the first immunotherapies. Since care teams were initially unprepared to manage colitis, many patients struggled to manage their now-worsening health and could have faced difficult complications – leading to stopping treatment.
Because side effects can be difficult to predict, oncology care teams may find it hard to inform patients about what to expect as they begin treatment. Patients will also need a highly structured way to report and manage their side effects between treatment doses, especially if they are choosing to self-administer therapies and will have fewer in-person appointments. The unpredictable and potentially severe or delayed toxicities also suggest a clear need to help patients understand what to do if things appear to be getting worse.
“Patients and care teams can clearly benefit from purpose-built digital health tools to help patients manage the administration of their therapies, monitor side effects and appropriately engage with their care teams between doses.”
PURPOSE-BUILT TECHNOLOGY TO SUPPORT IMMUNO-ONCOLOGY
Given the challenges of immuno-oncology, patients and care teams can clearly benefit from purpose-built digital health tools to help patients manage the administration of their therapies, monitor side effects and appropriately engage with their care teams between doses. This growing market of digital health solutions emphasises an engaging user experience and personalised interventions based on tested and approved clinical pathways.
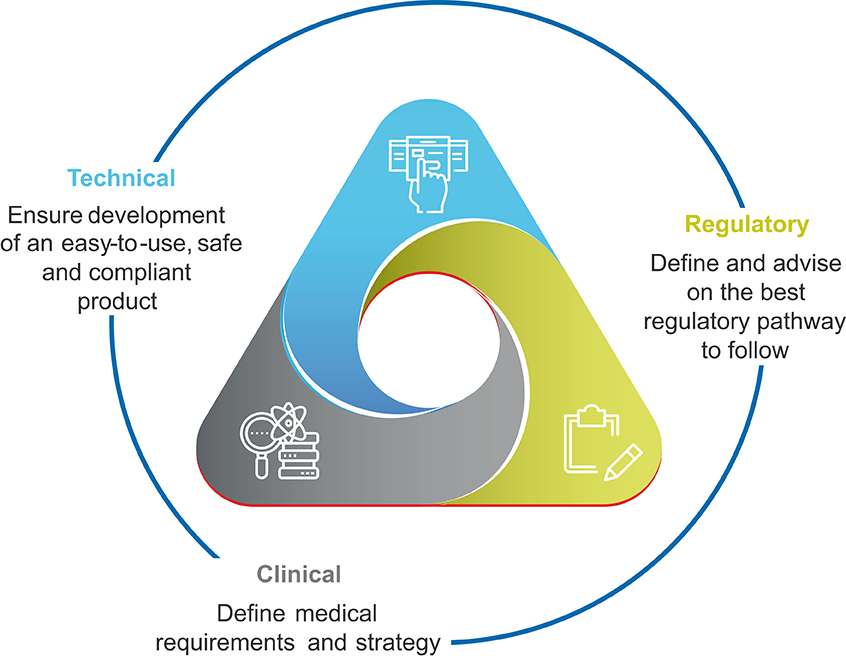
Figure 2: Team capabilities required to build technology to support immuno-oncology.
To create such a digital health tool, it is critical to bring together representatives from clinical, technical, pharmaceutical, medical device and regulatory and legal stakeholders from the beginning of the process (Figure 2). Patient input cannot be overlooked and should be gathered through both formative and summative studies; the former taking place throughout the design and development processes, with the latter functioning as a user test once a product is nearly finalised.
Creating such a multidisciplinary team brings some key advantages to the product development lifecycle. One is a collective understanding of the regulatory complexities that apply to digital health solutions. As these products are also held to different regulatory standards compared with traditional medical treatments, their value and safety can be demonstrated through randomised control trials (RCTs) and/or ongoing real-world evidence (RWE) generation. Applying this knowledge at the outset saves significant time in the long run, as issues are identified and addressed before significant redesigns are required.
Another advantage is the capability to test usability, efficacy and security throughout the product development lifecycle. Not only does this mirror best practices for software development, it also ensures that a product meets the needs of both patients and healthcare providers. Patients need an intuitive interface that is easy to navigate, while care teams should be spared a deluge of patient data and only receive notifications that require immediate attention, such as missed doses or worsening side effects.
With the right team in place, and with the right approach to design and development, a digital health solution can meet the needs of both patients and healthcare providers. In these situations, patients have the resources they need in a mobile app they can easily use, while clinical care teams receive the data and context they need to make the right care decisions on behalf of their patients.
PUTTING FORTH THE BEST PATIENT-FACING PRODUCT
It is critical that all patients can benefit from digital health support. This means designing products that present information viewable even to patients with limited vision, and to structure navigation menus and buttons to accommodate those with limited dexterity. At the same time, all digital solutions must strike a balance when it comes to sharing information with care teams. Too much information can be distracting, while too little leaves care teams uninformed about whether patients are making progress.
Organisations developing immuno-oncology digital products, such as Aptar Digital Health, need to account for the following factors.
Trustworthy Content: Patients are likely to have many questions about their immunotherapy, especially if it is their first time going through this type of treatment. Access to curated and approved resources will let patients educate themselves at their convenience, while giving care teams peace of mind that patients are only exposed to accurate and trustworthy information.
Self-Administration Guidance: Written resources alone may be insufficient to assist patients. Video tutorials work well to provide step-by-step instructions. Meanwhile, training devices can help patients get a feel for the proper angle and amount of pressure necessary for correct subcutaneous injection. They also allow for ongoing training in between doses, which helps patients build their confidence.
Self-Management Capabilities: Given the highly individualised nature of immuno-oncology, it is imperative that digital tools help patients monitor and measure their side effects. These capabilities should present information to patients in a familiar way, with an emphasis on clear language (and limited clinical terminology) and an ability to account for both physical and mental health.
Bidirectional Information Sharing: It is critical to keep care teams informed about a patient’s progress. If they report certain symptoms, or if they are having difficulty using an injection device, then it may be necessary to modify a treatment plan. Effective digital tools can provide this information to care teams at the appropriate moments in their clinical workflows to minimise disruptions and distractions.
Patient-Facing Decision Support: As patients routinely review educational content, administer therapies and record side effects, they are increasingly empowered to manage their care. Digital tools can further this empowerment with evidence-based decision-support tools. This is especially valuable in providing guidance to help patients decide whether the symptoms they are experiencing require a visit to the emergency department or may be resolved in another manner.
These considerations show that the goal of digital health solutions in immuno-oncology is to reassure patients that they are not alone. Tools that provide access to reputable resources, enable self-management and keep care teams in the loop help to educate, support and empower patients are critical as patients go through a difficult and disruptive care journey.
CONCLUSION
Immuno-oncology is a growing and promising field for immune checkpoint inhibitors. Regulatory bodies in the US, EU, Japan and elsewhere continue to approve new therapies in this field, while life science organisations are ramping up the trial of existing therapies for new indications and new formulations. Both steps will help to bring more highly personalised and targeted treatments to more patients around the world.
Increased use of immunotherapies makes it all the more imperative for the industry to develop purpose-built digital health solutions that support patient self-management. When these products go through well-thought-out design and development lifecycles that account for patient and provider needs and involve all key stakeholders from the very beginning, the result is a product that is integrated into the clinical workflow and aims to improve clinical outcomes and quality of life while further demonstrating the efficacy of immuno-oncology – and encouraging additional developments.
For more information on Digital Therapeutics, visit: www.aptar.com/pharmaceutical/digital-health-enhanced-patient-experiences.
REFERENCES
- “History of Cancer Treatments: Immunotherapy”. American Cancer Society, Jun 12, 2014.
- Hussein K, “Understanding the Role of Immuno-Oncology in Treating Cancer”. CancerCare, Aug 11, 2022.
- “Immunotherapy in Depth”. Web Page, Cancer Research Institute, accessed May 2023.
- Das S, Johnson D, “Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors”. J Immunother Cancer, 2019, Vol 7(1), p 306.


