To Issue 133
Citation: “Technology Showcase: CCBio’s Felice Dose”. ONdrugDelivery, Issue 133 (May 2022), pp 54–55.
In 2018, it was reported that approximately 50% of intravenous (IV) injections failed due to inflammation of the vein or phlebitis. To combat this, the pharma and biotech industries are looking for a new solution to ease patient pain. On-body injector (OBI) devices aim to respond to this need (Figure 1).
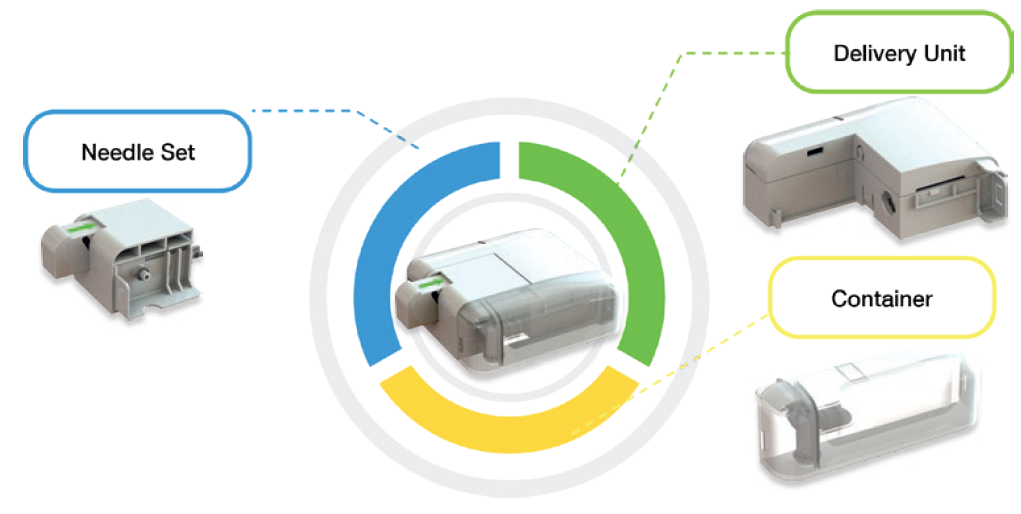
Figure 1: OBI devices are an answer to unmet needs in the pharma and biotech industries.
“Felice Dose provides a solution to the unmet needs present in the pharma industry, with the ability to deliver large drug volumes over variable- and long-duration injections.”
Recently, there has been a trend within pharma towards converting drugs formulated for IV delivery to a subcutaneous (SC) format. This trend is driven by a multitude of factors, including the long-term impacts of covid-19 and the potential offered by prefilled drug delivery options. SC formulations offer patients the option to self-administrate at home and will therefore likely replace IV formulations in many cases as IV administration must be performed in a clinic regardless of injection duration. It is expected that this trend towards SC administration will be especially prevalent in new biologic drugs based on therapeutic proteins. At-home administration would reduce the time burden of treatment on patients and healthcare providers significantly, often entirely eliminating the need for travel to and from the clinic.

Figure 2: CCBio’s Felice Dose.
As part of this trend, unconcentrated high-dose wearables OBI devices are emerging as a new category in the drug delivery market. OBI devices provide notable benefits for users and drug formulators, especially regarding drug concentration. However, the SC tissue’s extracellular matrix limits short-duration SC injection volumes to <2 mL in humans, according to hydrostatic and colloid osmotic pressure gradients, with delivery volumes >2 mL found to cause swelling and pain in skin if delivered too quickly. To counter this, OBI devices allow for longer injection durations; however, ensuring that patients can wear the combined weight of the high-dose drug and OBI device comfortably becomes a crucial design factor.
From the patient perspective, the most important need is receiving the correct drug volume accurately and the ability to activate the device easily. Additionally, regulatory scrutiny is increasingly important, with updates of the ISO 11608-6 series, especially those covering fluid lines and paths, meaning that meeting user needs and regulatory standards is the primary challenge faced by high-dose OBI devices.
To meet these challenges, CCBio presents Felice Dose (Figure 2), a delivery motor system-based OBI device ready for commercialisation. Felice Dose provides a solution to the unmet needs present in the pharma industry, with the ability to deliver large drug volumes over variable- and long-duration injections. The delivery motor system can adapt to the primary drug container, providing a very stable displacement unit for the drug, and the duration for filling and delivery can be programmed to suit the drug formulation. As such, the system can deliver a stable dose of drug per unit time under discontinuous pressure, reducing the swelling underneath the skin during injection and thus decreasing patient pain. Discontinuous pressure for a high-dose container is very important because otherwise, if a drug delivery system continually places a positive linear compression pressure on the container, pressure will accumulate and contribute to increased patient pain during the injection process.
Felice Dose’s delivery motor system is capable of handling various drug volumes, concentrations and viscosities, as well as different injection speeds and delivery times. The delivery motor system does not require continuous power, which has enabled CCBio to reduce the size of the battery and therefore the overall weight of the device. CCBio is currently testing the Felice Dose system for a 250 mL injection lasting up to 12 hours.
CCBio’s Felice Dose was successfully developed based on the perspective of both patients and pharma partners, ensuring that it contains all the necessary elements for an excellent OBI device. Felice Dose offers multiple options for customisation in terms of primary drug container, user interface and needle gauge and length. The Felice Dose primary drug container is extremely flexible – it can be configured to suit dose volumes of 5–250 mL (Figure 3) by using the mini-bag elastic polymer container system. The available needles include 6–8 mm of 29G steel needle coupled with a tube of soft needle that minimises the pain caused to the patient by the needle penetrating the skin.
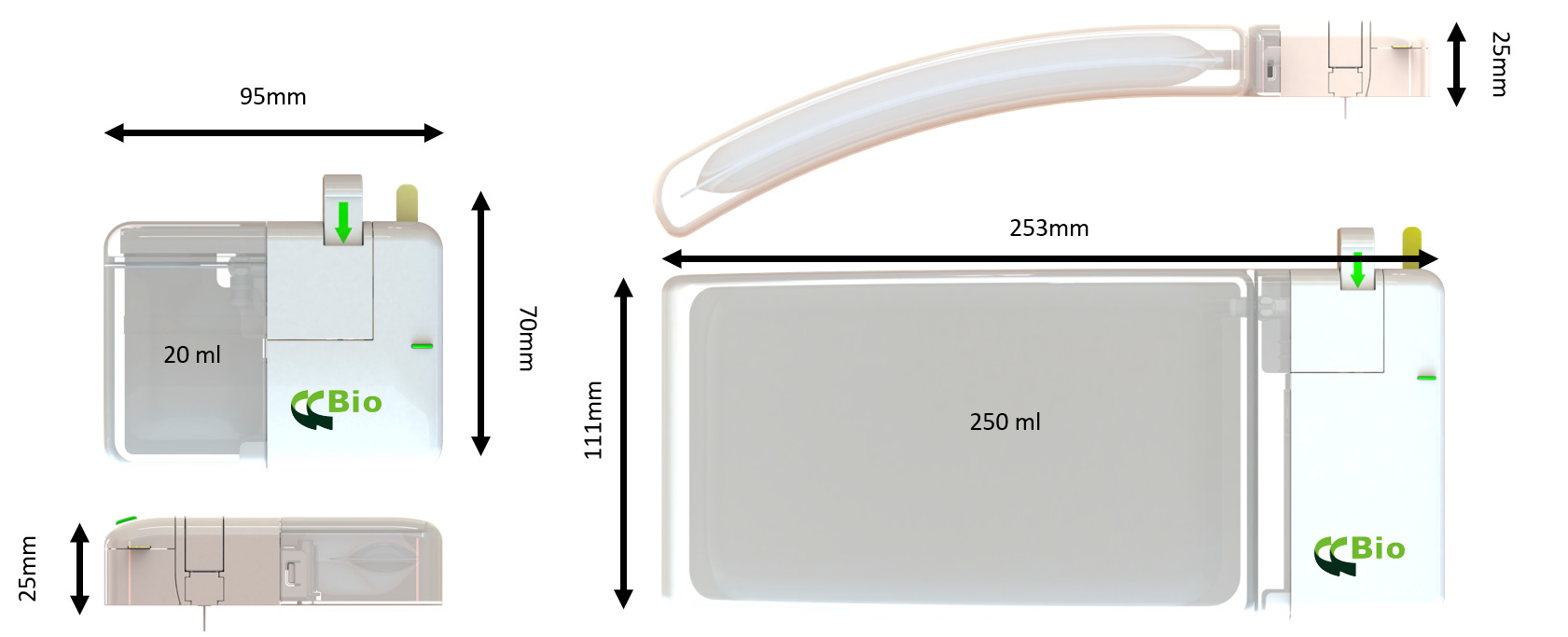
Figure 3: Felice Dose can be configured to fit delivery volumes of 5–250 mL.
The Felice Dose user interface includes options for WiFi, near-field communication (NFC) and Bluetooth connectivity, an LED display, fully-customisable programmability, a powerful delivery motor system and a lithium battery. The device’s smart program functionality can help patients take control of their treatment, making their daily life easier and more comfortable, and reducing the need for hospital visits and the involvement of healthcare providers.

