To Issue 152
Citation: Guasti M “Terumo Global Parenteral CDMO – Integrated Innovation”. ONdrugDelivery, Issue 152 (Oct 2023), pp 119–122.
Michele Guasti discusses the advantages that Terumo’s parenteral CDMO brings to customers due to its position as a single service provider from formulation to final product and its intimate understanding of Terumo’s range of prefillable polymer syringes.
Working with Terumo’s parenteral contract development and manufacturing organisation (CDMO) brings customers a uniquely integrated service offering. Today’s Terumo combines its high-quality polymer prefillable syringe manufacturing capabilities together with its complete CDMO service offering, which includes formulation development, fill-finish services, device assembly and packaging, all in one trusted partner. For Terumo’s customers, integrated innovation means end-to-end services that deliver injectable products of the highest quality, compliance and with reduced time to market.
“Having recently expanded its CDMO offering to biopharmaceutical companies around the world, Terumo has positioned itself to meet global industry requirements successfully.”
THE TERUMO SYRINGE EXPERIENCE
Terumo started producing its first disposable plastic syringes in 1963 and has continued to invest in the latest syringe technologies ever since. Glass is historically known for its chemical inertness and therefore widely used as the primary material for prefillable syringes. Terumo recognised the limitations of glass syringes and developed new, innovative prefillable polymer syringes with higher resistance to breakage and dimensional accuracy for the pharmaceutical market.1
Terumo has continued to innovate by bringing together its industry-leading polymer syringes with advanced formulation and sterile fill-finish services as an integrated CDMO. For over two decades, Terumo has been recognised for the quality and service standards it provides to many of Japan’s leading biopharmaceutical companies for prefilled syringe (PFS) CDMO development and manufacturing.
THE TERUMO CDMO DIFFERENCE
Having recently expanded its CDMO offering to biopharmaceutical companies around the world, Terumo has positioned itself to meet global industry requirements successfully. Terumo conforms to international quality and regulatory standards in its three modern and efficient sterile manufacturing and development sites, all located in Japan. Combining the global standard in polymer PFS supply with exceptional sterile formulation development services through a uniquely co-ordinated approach provides significant advantages to Terumo’s CDMO customers.
By working as a single supplier with an unmatched understanding of the capabilities of its own syringes, Terumo is able to select the best device for each individual project. It also allows Terumo’s CDMO teams to co-ordinate the formulation and device development steps and to arrive at an optimal formulation quickly, considering all the various challenges presented by potential interactions between the drug and the device. Terumo’s highly efficient sterile filling operations use modern, flexible and highly automated sterile filling lines suitable for both clinical and commercial manufacturing volumes.
The result is that Terumo’s integrated innovation approach can significantly de-risk a project, enabling accelerated timelines. Terumo’s CDMO services span the full range of services required to take a customer’s molecule from drug formulation to fully packaged commercial product, all with a single partner (Figure 1).
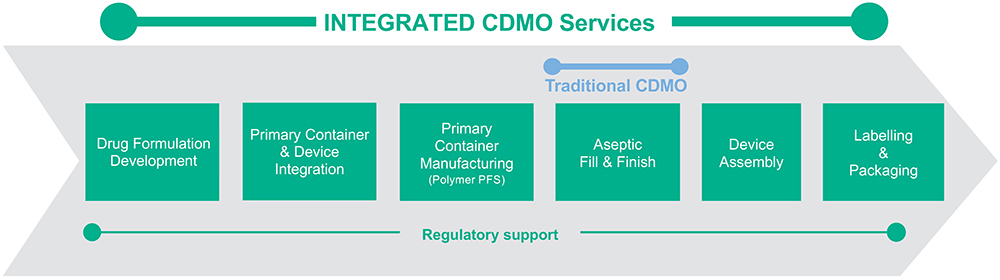
Figure 1: Terumo’s integrated CDMO services.
“The practicalities of scale-up, technology transfers and regulatory filings are all incorporated into development programmes by Terumo’s experienced scientists from the beginning.”
TERUMO’S SERVICE CAPABILITIES PROFILE
Drug Formulation Development
Terumo has been developing and producing sterile liquid formulations for a range of medical applications since the 1980s. For over 20 years, Terumo’s skilled scientists have applied their sterile injectable formulation development knowledge to create fully optimised and scalable PFS products on a contract basis. Terumo applies a multidisciplinary approach involving a team of pharmaceutical scientists who apply their specialised knowledge in drug stability, delivery systems, pharmacokinetics and primary container drug interactions. The company’s PFS development capabilities are fully supported with specialised analytical capabilities that can be used to study chemical composition, molecule characteristics, excipient selection and reactivity.
Terumo’s biochemistry and biotechnology experts leverage their knowledge of drug-target interactions and molecular biology techniques to produce stable, functional formulations of recombinant proteins or monoclonal antibodies. The practicalities of scale-up, technology transfers and regulatory filings are all incorporated into development programmes by Terumo’s experienced scientists from the beginning.
Taking an integrated approach to both the syringe selection and formulation design is critical to advancing new PFS products into manufacturing efficiently. Terumo, as a CDMO, is uniquely positioned to achieve this and pass the benefits onto its customers.
“Terumo’s integrated CDMO services are performed to the company’s strict quality standards, producing the highest quality products for customers.”
Primary Container and Device Integration
Any PFS product development programme must consider drug–container interactions. Terumo scientists optimise formulations to avoid undesirable interactions with syringe body, plunger and lubricant materials that may impact extractable/leachable specifications, as well as overall product stability. When considering primary container and device selection for sterile injectable products, Terumo’s integrated CDMO services assess a range of product parameters, including:
- Material selection – contact material inertness with formulation
- Primary container design – dosing, drug delivery and user factors for the primary container both for the syringe alone and in combination with an injection device, such as an autoinjector
- Compatibility – factors such as sensitivity to light, oxygen, moisture, silicone oil, glue, tungsten, sterilisation impact, specialised container materials and coatings
- Drug stability – primary container impact on drug formulation stability using realtime and accelerated stability studies.
Polymer PFS Supply
Terumo has been a well-established supplier of advanced polymer PFSs to the biopharmaceutical industry for many years. Terumo’s range of polymer PFSs and components are known for excellent strength and clarity, as well as tight dimensional tolerances, contributing to a lower overall cost of ownership for customers. With ready-to-fill PLAJEX™ cyclo-olefin polymer syringes designed to meet the needs of a wide variety of drug types, including those requiring low reactive containers,2 Terumo offers the syringe options necessary for protecting even the most sensitive biotherapeutic molecule requirements.3 Some molecules can be sensitive to standard syringe coatings and lubricants, so PLAJEX™ mitigates issues related to silicone oil, providing superior molecule protection and minimising the risk of protein aggregation.3
Terumo’s R&D scientists have also designed needles for better injectability without compromising patient comfort for their polymer syringe lines.4 With Terumo, needle design and syringe features, specially designed for different product routes of administration, syringe formats and drug delivery systems, can be customised to a degree that is not easily possible when using separate suppliers for each outsourcing step.
Aseptic Fill-Finish Capabilities
Custom formulations, paired with the ideal Terumo syringe, can be seamlessly transferred into Terumo’s network of sterile manufacturing facilities, whether it is for clinical or commercial-scale production. With three sterile manufacturing locations in Japan, Terumo offers high-quality aseptic filling or sterile fill-finish with terminal sterilisation capacities to meet virtually any demand. The company’s sterile filling lines are designed to minimise the risks of human interaction throughout the process by operating under full isolator protection with highly automated controls. Terumo’s highly automated lines can handle everything from container moulding to aseptic filling, sealing and packaging, all while meeting the highest regulatory standards.
Device Assembly
Some CDMO customers require their PFS product to be assembled as part of a final safety or autoinjector device. Terumo’s CDMO team perform the final device assembly under controlled conditions, with the additional autoinjector or safety device provided by the customer or sourced by Terumo from a third-party provider. The final device must be designed to accommodate Terumo’s PFSs but can be included in a manual or fully automated process to deliver the final combined product, ready to ship.
Labelling and Packaging
Every product must be appropriately labelled and packaged to meet the local market’s regulatory requirements. Terumo can offer comprehensive labelling and packaging services in a variety of final formats that comply with all packaging regulations in most major markets. Terumo operates highly automated packaging and precision kitting services to meet a wide range of needs. Labelling and packaging specifications can be defined in parallel with development activities, shortening the timeframe needed to bring the product to market.
“Considering that Terumo was founded on developing leading-edge medical products and services, it is easy to see why innovation is such a big part of the company’s heritage and way of thinking.”
TERUMO QUALITY
Terumo’s integrated CDMO services are performed to the company’s strict quality standards, providing customers with reassurance of compliance with all relevant standards. The company applies the same quality management system principles and adheres to applicable regulatory requirements across all its operations.
Terumo’s expertise in quality control and quality assurance gives customers the confidence that their product will meet regulatory standards and specifications wherever they are used. The company’s knowledge of analytical techniques, stability testing and compliance with good manufacturing practices (GMP) all contribute to producing high-quality products. Terumo’s Japanese manufacturing sites (Figure 2) are GMP-compliant and certified by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). Both of the company’s aseptic fill-finish sites have been successfully inspected by the US FDA and the EMA has visited one site for a commercial product inspection. This track record of success with leading international regulatory bodies demonstrates Terumo’s continuing commitment to achieving the highest quality standards for its PFS CDMO customers.
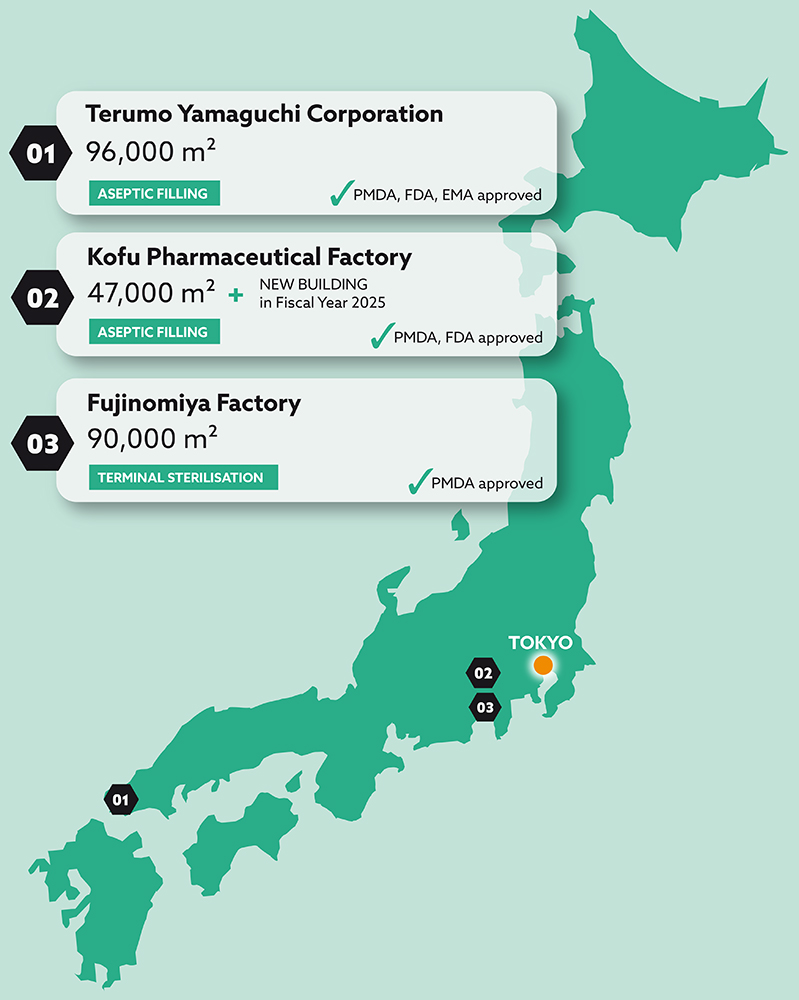
Figure 2: Map and locations.
BENEFITS OF INTEGRATED
INNOVATION Terumo’s integrated CDMO services offer substantial benefits to its customers, including:
- One partner – end-to-end service offering from formulation to final packaged product
- Quality – quality systems that use the latest technologies and compliant processes to build quality into every aspect of its operations
- Regulatory compliance – decades of experience compliantly engineering and manufacturing combination drug products and devices
- Reduced cost of ownership – lower investment cost to develop new molecules when working with a single supplier
- Time to market – a single supplier accelerates supplier qualification, validation and communication
- Supply chain simplification – one single source for device technology and drug product development and manufacturing services, from small clinical batches to commercial-scale production
- Consulting and engineering support – support provided through development plans, regulatory applications and high-value-added products.
Terumo’s integrated approach to offering complete, single-source CDMO services for PFSs is innovative. Considering that Terumo was founded on developing leading-edge medical products and services, it is easy to see why innovation is such a big part of the company’s heritage and way of thinking. Terumo has always sought to create solutions to the challenges its customers face. Decades of expertise were applied to develop Terumo’s industry-leading polymer based syringes that have since become the global industry standard.
Terumo’s innovative proprietary silicone oil-free containers, in combination with i-coating™ technology (chemically bonded coating) present on the stoppers, can mitigate issues related to silicone oil, providing superior molecule protection.3 All of these technological innovations came from Terumo’s scientists and engineers. Now, the same dedication to innovation through science is being applied to the company’s end-to-end CDMO services, and it is the customers and patients who benefit most.
To find out more, visit: www.terumopharmaceuticalsolutions.com.
REFERENCES
- “The Gold Standard of High-Purity Plastic”. Web page, ZEONEX, accessed Sep 2023.
- “Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products”. US FDA, Aug 2014.
- Yoshino K et al, “Functional evaluation and characterization of a newly developed silicone oil-free prefillable syringe system”. J Pharm Sci, 2014, Vol 103(5), pp 1520–1528.
- “Advantages of a tapered needle for use in pre-filled biopharmaceutical products”. Internal study, Terumo, Mar 2019.
Previous article
INTERVIEW: DOUG BRYANS, BRYLLAN & JOHN MERHIGE, CREDENCENext article
AUTOMATING ASSEMBLY TO EXPEDITE TIME-TO-MARKET
