To Issue 154
Citation: Dantas C, Krueger U, “The Importance of the Breathing Manoeuvre in Nebulised Therapies”. ONdrugDelivery, Issue 154 (Nov 2023), pp 18–20.
Carolina Dantas and Ulf Krueger highlight the importance of the breathing manoeuvre in nebulised therapies – and describe a small study designed to test the effectiveness of patient feedback technology when it comes to guiding the inhalation manoeuvre.
Nebulised therapies, by delivering medication directly into the lungs, have been essential in the treatment of respiratory diseases such as asthma, chronic obstructive pulmonary disease and infections. The breathing manoeuvre plays a pivotal role in drug deposition within the respiratory system, particularly when aiming for deep lung penetration and minimal upper airway impaction.
“When using a nebuliser, spontaneous breathing patients naturally perform different breathing manoeuvres, with a wide variability of
flow rate, respiratory rate or inhaled volume.”
THE BREATHING MANOEUVRE AND PULMONARY DRUG DEPOSITION
Several studies have investigated the main influencing parameters in pulmonary drug deposition: the airway structure, the aerosol characteristics and the breathing manoeuvre.1,2 Targeted lung deposition is an important concept in nebulised therapies, and it is defined by the intentional manipulation of one or more of these parameters to promote aerosol deposition in certain locations in the respiratory system.
When using a nebuliser, spontaneous breathing patients naturally perform different breathing manoeuvres, with a wide variability of flow rate, respiratory rate or inhaled volume. Therefore, the aerosol deposition – and consequently the dose – varies considerably among patients. So, how can this variability be reduced? According to Brand et al, by controlling the breathing manoeuvre. With slow inhalations, at low flow rates, a drug can be targeted to the lung periphery with high efficacy and low variability. Additionally, to achieve a higher alveolar deposition, it is also important to consider the need for higher inhaled volumes.
Overall, the inhalation manoeuvre of the patient has a major relevance for the amount of drug that will deposit deep into the lungs.2 When considering the strong influence of the particle size and the breathing manoeuvre, there seems to be an “opportunity window” when lung deposition is maximised. This, in particular for nebulised therapies, allows the development of devices that provide some control over targeted lung deposition.3
NEBULISATION – CONTINUOUS OR BREATH TRIGGERED?
There are several types of nebulisers in the market, and these are frequently categorised according to their mechanism of action into three main types: jet nebulisers, ultrasonic nebulisers and vibrating mesh nebulisers. Considering the importance of the breathing manoeuvre, nebulisers can also be differentiated by their nebulisation mode. Some devices deliver aerosol continuously during the entire breathing cycle (inspiration and expiration). Aerosol waste can be reduced by storing the aerosol generated during exhalation in a reservoir, and subsequently releasing it during inhalation, allowing optimisation of the nebuliser performance. On the other hand, breath-triggered/activated/actuated devices deliver aerosol only during the inspiratory phase using a breath actuation mechanism triggered by the patient.
Besides the nebulisation mode, devices can have different technologies to control or guide the breathing manoeuvre of the patient, which were developed to increase the aerosol delivery efficiency. These include, for example:
- Software that predicts patient inhalation based on the previous breathing cycles
- Delivery of aerosol bolus at a precise point in the inspiration phase
- A resistance mechanism to regulate the inhalation flow
- Feedback systems to support and guide the patient.
Ultimately, these different nebulisation modes and technologies represent specific features of each existing device. Understanding the impact on respiratory drug delivery is critical for minimising medication waste, enhancing patient adherence and experience, and achieving better therapeutic outcomes.
A STUDY ON FEEDBACK TECHNOLOGY TO GUIDE INHALATION
Patient feedback technology enables nebulisers to provide guidance for patients to perform an ideal breathing manoeuvre according to the targeted lung deposition area. Haptic feedback, for example, provides tactile sensations or vibrations to the user for improved interaction or guidance. The same applies to other forms of feedback – for example, digital, visual, auditory or thermal.
This article describes a small study aimed at validating the feedback system of the Kolibri mesh nebuliser in guiding inhalation during the nebulisation of 1 mL of saline. The inhalation flow rate threshold was defined as 15 L/min. Therefore, below that threshold a positive and pleasant vibration instructed the user to maintain the inhalation flow rate, and above the 15 L/min threshold, a negative and unpleasant vibration occurred. Eight healthy subjects were included in the study and data on inhalation flow rate (in L/min) and duration (in seconds) were collected and analysed (see Figure 1).
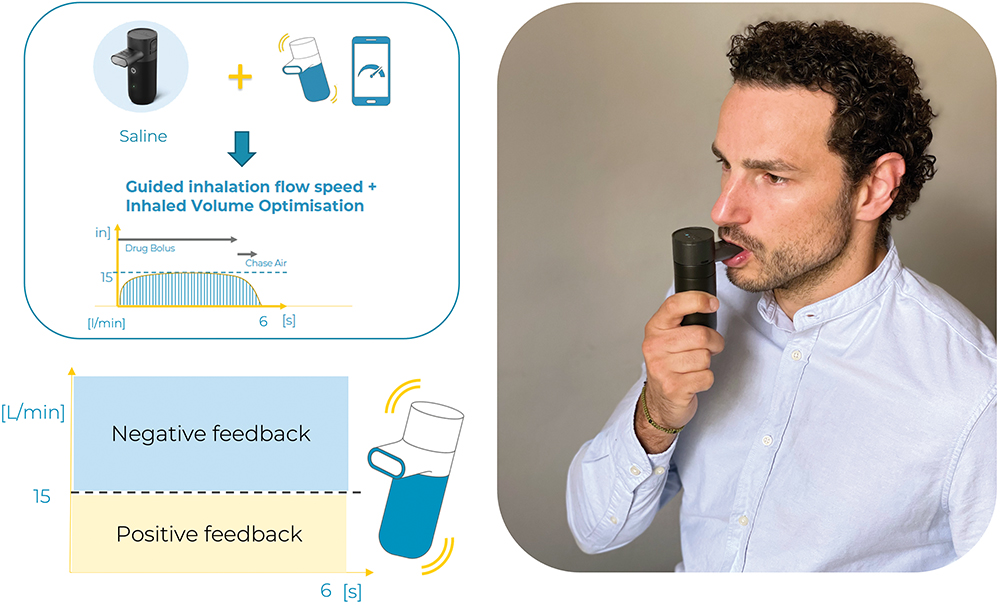
Figure 1: Study protocol, feedback settings and picture from participant (authorised).
“Patient feedback technology enables nebulisers to provide guidance for patients to perform an ideal breathing manoeuvre according to the targeted lung deposition area.”
In the individual analysis, each inhalation curve was registered, as well as the number of inhalations needed to nebulise 1 mL of saline (see Figure 2). Such detailed tracking allowed the determination of the inhalation variability of each participant. Better inhalation manoeuvres reduced the amount of time necessary for nebulisation, resulting in higher efficiency.
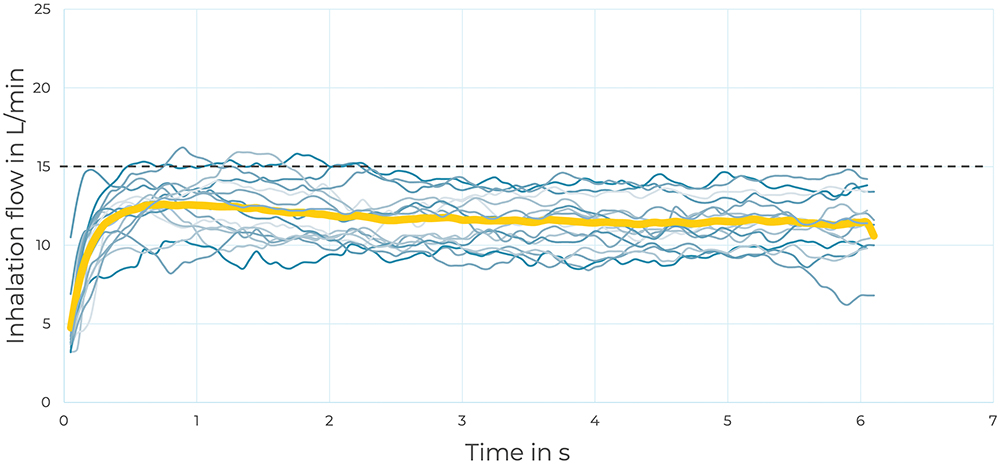
Figure 2: Example of subject 6 guided inhalation flow rate curves. Each inhalation is registered in blue and the average in yellow.
The group analysis showed that all the participants could easily follow the feedback and maintain the inhalations below the threshold of 15 L/min, as illustrated in Figure 3. As a group, there was little variability in the inhalation manoeuvres, with an average of 16%. Additionally, two participants illustrated a learning curve process – revealing their ability to recognise the different types of haptic feedback and progressively learn to follow the desired inhalation flow rate.
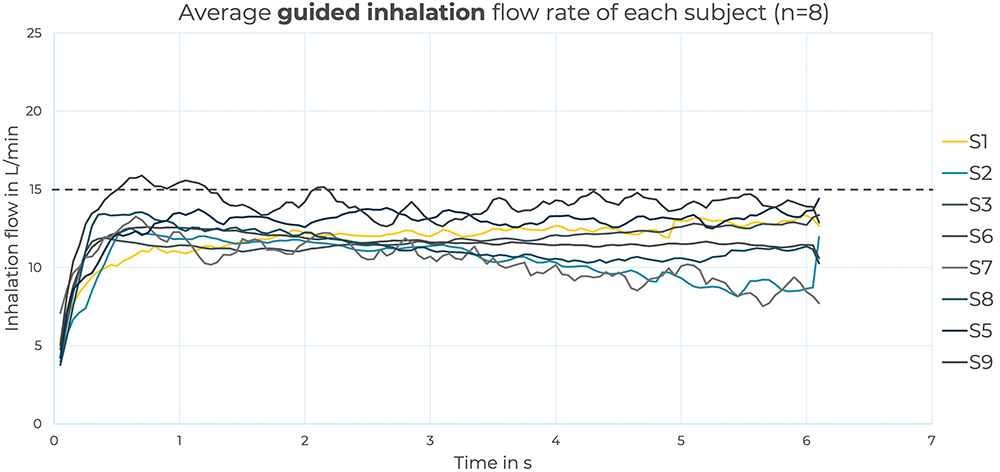
Figure 3: Group analysis, in which each curve represents the average inhalation flow rate of the eight participants. Overall, the majority of the participants could maintain the inhalation flow below the 15 L/min defined threshold.
“This small study demonstrated the ability of feedback technology to effectively guide the inhalation manoeuvre during nebulisation.”
This small study demonstrated the capacity of feedback technology to effectively guide the inhalation manoeuvre during nebulisation. Using haptic feedback, it was possible to reduce the variability typically associated with spontaneous breathing nebuliser users. Future larger studies assessing the effect of feedback-guided inhalation on targeted lung deposition will provide further valuable data.
In nebulised therapies, the breathing manoeuvre plays a major role in achieving a targeted drug deposition and consequently the best treatment outcomes. Understanding and tackling the challenge to reduce the breathing manoeuvre’s variability is key. Current technologies, in particular breath-triggered nebulisers with guided inhalation systems, provide opportunities to further enhance aerosol lung deposition. Comprehensive studies in the future will shed light on the impact of feedback-guided inhalation on targeted lung deposition, ultimately advancing the field of respiratory therapies towards more personalised and effective treatments.
REFERENCES
- Brand P et al, “Total Deposition of Therapeutic Particles during Spontaneous and Controlled Inhalation”. J Pharm Sci, 2000, Vol 89(6), pp 1–8.
- Scheuch G et al, “Clinical perspectives on pulmonary systemic and macromolecular delivery”. Adv Drug Deliv Rev, 2006, Vol 58, pp 996–1008.
- Haeussermann S, Sommerer K, Scheuch G, “Regional Lung Deposition: In Vivo Data”. J Aerosol Med Pulm Drug Deliv, 2020, Vol 33(6), pp 291–299.

