To Issue 170
Citation: Rogueda P, “Transforming Mental Health Care: The Rise of Inhalable Psychedelics?”. ONdrugDelivery, Issue 170 (Apr 2025), pp 43–49.
Philippe Rogueda discusses how inhaled psychedelic therapies are emerging as a potential game-changer in mental health due to their fast onset and precise dosing, and how their potential has resulted in a growing pipeline of therapies, paving the way for a new era in mental healthcare.
In 2022, the WHO published its largest review of world mental health since the turn of the century. It urged all countries to accelerate the implementation of the “Comprehensive Mental Health Action Plan 2013–2030”, including strengthening care by changing where, how and by whom mental health care is delivered and received.
The issue of global mental health has driven research for faster-acting, more effective treatments, with growing recognition that traditional psychiatric medications often fail to meet the needs of patients. Many antidepressants take four to six weeks to reach full efficacy, and only approximately 30% of patients with major depressive disorder (MDD) achieve remission after first-line treatment. For those with treatment-resistant depression (TRD), post-traumatic stress disorder (PTSD) and severe anxiety disorders, the limitations of existing therapies can be life-threatening.
“WITH 18 INHALABLE PSYCHEDELIC DRUGS CURRENTLY IN DEVELOPMENT, THE SECTOR IS GAINING MOMENTUM AS PHARMACEUTICAL COMPANIES, BIOTECH FIRMS AND DRUG DELIVERY SPECIALISTS EXPLORE ITS POTENTIAL.”
Could innovations in inhaled psychedelic therapies (iPTx) be the breakthrough solution? With 18 inhalable psychedelic drugs currently in development, the sector is gaining momentum as pharmaceutical companies, biotech firms and drug delivery specialists explore its potential. Inhalable therapies can deliver measurable effects within minutes, making them well-suited for treating acute psychiatric episodes, rapid intervention in suicidal ideation and emergency mental healthcare.
GAINING MOMENTUM IN PSYCHEDELIC DRUG DEVELOPMENT
Over the past decade, psychedelics have transitioned from countercultural obscurity to cutting-edge pharmaceutical research. Between 2018 and 2024, according to GlobalData’s clinical trials database, the number of clinical trials investigating psychedelic therapies increased by 137% – from 48 to 114 – reflecting a surge of interest in their potential. The global psychedelic drugs market is predicted to surpass US$8 billion (£6.3 billion) by 2030, supported by a 15% compound annual growth rate.
“UNLIKE TRADITIONAL ANTIDEPRESSANTS, WHICH REQUIRE CHRONIC DAILY USE, MANY PSYCHEDELIC TREATMENTS EXERT LONG-LASTING EFFECTS AFTER JUST A SINGLE SESSION – AN IMPORTANT DISTINCTION THAT COULD TRANSFORM HOW DEPRESSION AND PTSD ARE MANAGED.”
While much of this growth has been driven by oral and intravenous psychedelic formulations, inhalable therapies are gaining traction as an effective alternative. Formulations for nasal sprays and soft mist inhalers (SMIs), for example, offer good bioavailability, fast onset and precise dosing, positioning them as potential game-changers in psychiatric medicine. Unlike traditional antidepressants, which require chronic daily use, many psychedelic treatments exert long-lasting effects after just a single session – an important distinction that could transform how depression and PTSD are managed.
THE FIRST MAJOR iPTx SUCCESS: SPRAVATO’S MARKET IMPACT
The first inhalable psychedelic to receive regulatory approval was Johnson & Johnson’s Spravato (esketamine) nasal spray, which the US FDA approved in 2019 for TRD, indicated for patients who have taken two or more oral antidepressants and still experience symptoms.1 For the first four weeks, Spravato is taken twice a week under the supervision of a healthcare provider. After that, it is typically taken once a week. In clinical trials, the most significant reduction in depressive symptoms was seen at 24 hours after first use.
By offering rapid symptom relief, Spravato has proven that iPTx can be a viable, scalable treatment option. Its commercial impact has been significant, with sales reaching $689 million in 2023 with forecasts signalling major growth over the decade (Figure 1). This has not only demonstrated strong market demand but has also encouraged greater investment in nasal spray and inhalation-based psychedelic treatments.
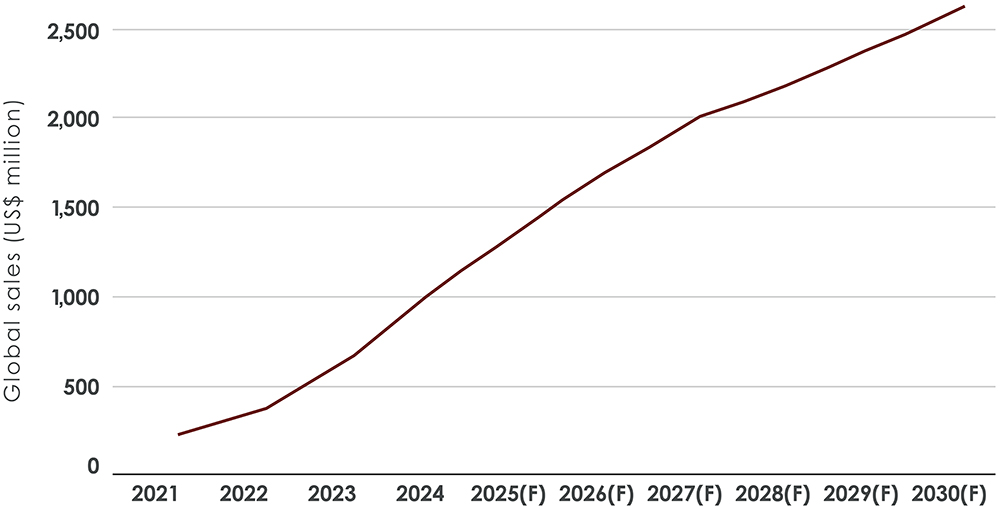
Figure 1: In 2030, total Spravato sales are forecast to reach $2.6 billion.
Spravato’s success has also paved the way for regulatory progress. Historically, regulatory agencies have been hesitant to approve psychedelic-based medicines, but Spravato’s performance has set an important precedent, providing a framework for future psychedelic inhalation therapies.
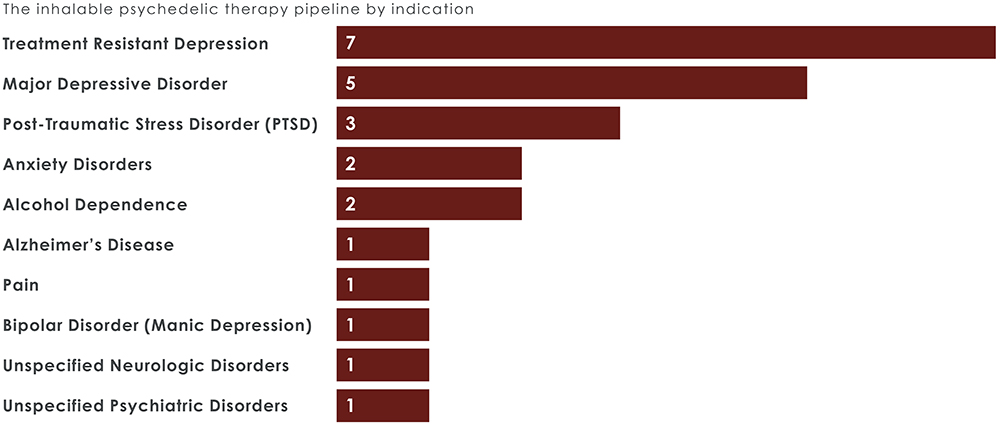
Figure 2: Most inhaled psychedelics in development are indicated for depression.
iPTx IN 2025: WHAT IS IN THE PIPELINE?
With 18 inhalable psychedelic therapies in development, drug makers are targeting various psychiatric and neurological conditions, with depression, PTSD, anxiety disorders, addiction treatment and neurodegenerative diseases among the key areas of focus (Figure 2). The expanding pipeline indicates growing confidence in the potential of iPTx, with several therapies expected to enter late-stage trials within the next five years (Figure 3).
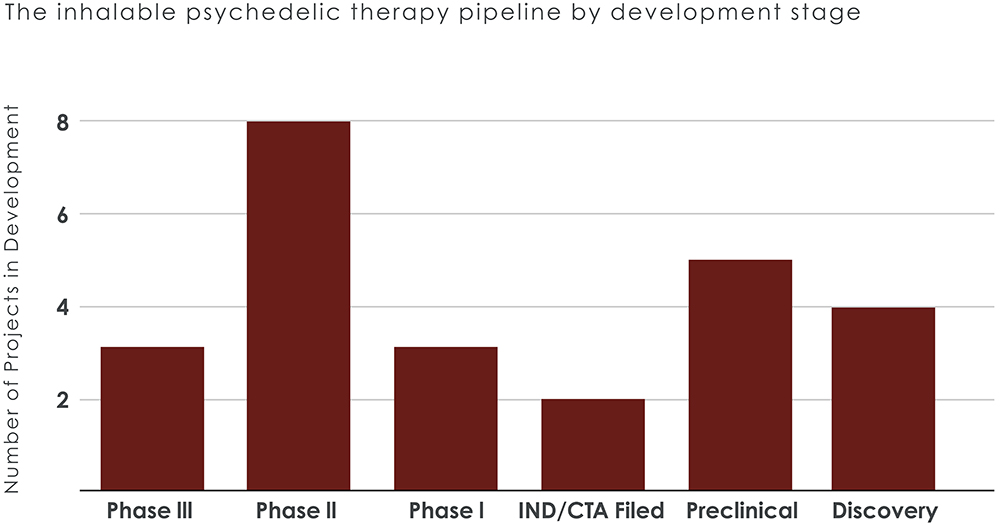
Figure 3: The largest portion of the pipeline is in Phase ll development.
Ketamine-Based Compounds
Beyond esketamine, there are further ketamine-based compounds demonstrating therapeutic potential. Currently in clinical trials for MDD and PTSD, ketamine hydrochloride is a racemic mixture of ketamine that has been used for anaesthesia, pain management and psychiatric disorders. It acts as an N-methyl-D-aspartate (NMDA) receptor agonist and has been under development with intranasal formulations in the US and China.2
Meanwhile, as a combination therapy with intranasal administration, ketamine with sufentanil enhances ketamine’s antidepressant and analgesic effects. Sufentanil is an opioid analgesic and this combination is being investigated by a Danish pharmaceutical company in a Phase III paediatric trial for pain management.3
A pharmaceutical company based in Florida (US) is also developing ketamine based drugs for intranasal delivery. SPC-14 is a preclinical-stage combination therapy being investigated for Alzheimer’s disease.4 The ketamine acts by targeting NMDA receptors, while the other API targets 5-hydroxytryptamine receptor 4, potentially treating the cognitive and neuropsychiatric symptoms of the disease.
Tryptamine-Based Compounds
Fast-acting dimethyltryptamine (DMT) acts as a 5-hydroxytryptamine receptor 2 agonist, which can induce profound alterations in perception and cognition. Inhaled DMT formulations are currently being developed for MDD and PTSD, using its ability to promote neuroplasticity. There are two inhalable DMT formulations currently in development in Canada, one being a liquid inhaled formulation indicated for TRD that is supported by positive initial results in a Phase II trial.5
5-methoxy-DMT (or mebufotenin) is a potent short-acting psychedelic found in certain plant species and toad venoms. Synthetic formulations have shown its potential for treating depression, anxiety and substance-abuse disorders by rapidly modulating serotonin receptors and inducing therapeutic dissociative states. At least two companies are currently working on clinical-stage inhalable mebufotenin drugs. One lead candidate is currently being examined in a Phase II trial for MDD and TRD, having already completed open-label Phase II trials in patients with bipolar II disorder and postpartum depression. The candidate is formulated for inhalable administration.6
Meanwhile, a British biotech has opted for nasal administration of its mebufotenin candidate, which is now in Phase II trials. It has completed an open-label study in patients with alcohol dependence and is recruiting for studies in patients with MDD and TRD. Findings from an open-label Phase IIa study in TRD patients were positive – a single 10 mg dose had a rapid antidepressant effect, with 55% of patients demonstrating a 50% or greater improvement in depression symptoms the following day.7
In 2024, a Canadian biotech raised $150 million to progress two clinical-stage programmes with inhaled drug delivery.8 This news came in the same month that the FDA granted breakthrough therapy designation to a deuterated psilocybin analogue with a similar structure to neurotransmitters such as serotonin. It has now initiated a Phase III programme in participants with moderate-to-severe MDD and plans to initiate a second pivotal study in the first half of 2025.
“THE STUDY REVEALED A SIMILAR ONSET OF EFFECT AND DOSE PROFILE TO INTRAVENOUS DMT, YET THE INHALED FORMULATION HAD AN APPROXIMATELY 300% LONGER DURATION OF ACTION AND MORE THAN A 40% IMPROVEMENT IN ITS BIOAVAILABILITY.”
Its second candidate is an inhalable deuterated DMT designed for anxiety disorders that is currently being evaluated in a Phase II study. It has a shorter onset and duration than traditional psychedelics, making it well-suited for controlled therapeutic settings. In 2022, it published pharmacokinetic data from preclinical studies demonstrating the benefits of delivering its deuterated DMT molecule via inhalation. Specifically, the study revealed a similar onset of effect and dose profile to intravenous DMT, yet the inhaled formulation had an approximately 300% longer duration of action and more than a 40% improvement in its bioavailability.9
THE ROLE OF DRUG DELIVERY INNOVATION
The effectiveness of inhalable psychedelics depends not only on the therapeutic compounds themselves but also on the precision of their delivery systems. Unlike traditional psychiatric medications, which can be swallowed, injected or infused intravenously, these novel therapies require specialised inhalation devices to ensure consistent dosing.
This is where Merxin Ltd plays a crucial role. As a leader in inhalation technology, the company designs high-performance drug delivery devices that optimise patient experience and therapeutic efficacy. By ensuring that pharmaceutical innovators have access to next-generation inhalation platforms, Merxin Ltd is shaping the future of inhalable psychedelic treatments. The company’s product portfolio includes devices such as MRX004 SMI, designed to deliver fine mist sprays for efficient drug absorption, and MRX006 multidose dry powder inhaler, which operates on an open-inhale-close mechanism, making it suitable for various therapeutic applications.
FUTURE OUTLOOK FOR iPTx
The potential behind inhalable psychedelics is undeniable. The success of Spravato, combined with a growing pipeline of new therapies, suggests that psychiatric treatment could be on the verge of a major transformation. By offering rapid symptom relief, precise dosing and improved bioavailability, these therapies have the potential to redefine mental health treatment.
As investment, research and regulatory acceptance continue to grow, inhalable psychedelics are poised to reshape the future of mental health treatment. For pharmaceutical companies, biotech firms and drug delivery specialists, the shift towards iPTx presents significant commercial opportunities. By harnessing advanced inhalation technologies and partnering with inhalation device industry leaders, such as Merxin Ltd, pharmaceutical companies can position themselves at the forefront of this nascent market.
REFERENCES
- “Spravato (esketamine)”. Patient Information Leaflet, Janssen, 2024.
- “About SLS-002 (intranasal ketamine)”. Web Page, Seelos Therapeutics, accessed Apr 2025.
- “Cessatech announces superior simulated pain efficacy in children favouring its lead candidate CT001 relative to its active comparators”. Press Release, Cessatech, Jun 2024.
- Floersh H, “Silo Pharma’s ketamine-based drug for Alzheimer’s effective against dementia symptoms in mice”. Fierce Biotech, Jan 2024.
- “Biomind Labs Announces Positive Initial Results From Its Phase II Trial on Its BMND01 Candidate for Treatment Resistant Depression”. Press Release, Biomind, 2022.
- “Our Pipeline”. Web Page, GH Research, accessed Apr 2025.
- “Beckley Psytech announces positive initial data from Phase IIa study of novel 5-MeO-DMT formulation BPL-003 for Treatment Resistant Depression”. Press Release, Beckley Psytech, Mar 2024.
- “Cybin Announces Closing of the Oversubscribed Private Placement of US $150 Million”. Press Release, Cybin, Mar 2024.
- “Cybin Announces Positive CYB004 Data Demonstrating Significant Advantages Over Intravenous and Inhaled DMT”. Press Release, Cybin, Apr 2022.

