To Issue 164
Citation: Moakes G, Sadi T, “Trust the Progress: Introducing Automation to Enhance On-Body Injector Manufacturing” . ONdrugDelivery, Issue 164 (Sep 2024), pp 54–58.
Greg Moakes and Tzach Sadi describe the process and benefits of automating the production process for LTS‘s on-body injector device, Sorrel™.
From the athlete striving for marginal performance gains to the business seeking energy consumption savings, the notion of continual improvement is a prerequisite for sustaining progress Within manufacturing environments, this philosophy has long been crystallised in the “Kaizen” approach, which emerged in the 1980s as an articulation of a series of tactical methods used by businesses in Japan (most notably Toyota) to enhance their efficiency, value and effectiveness. Continual improvement can provide the means to address particular issues and difficulties, such as downtime and delays. Alternatively, it can provide a platform to unlock previously hidden enhancements in areas such as quality and speed.
The same is true of drug delivery device manufacturing. In this world, the stakes for continual improvement are extremely high, not only in terms of ensuring optimal quality but also regarding “future proofing” the supply chain in the face of the growing demand, which, today, is driven in large part by increasing patient preference for on-body solutions.
“The philosophy of constant improvement is embedded in LTS’s thinking across product design, development and manufacture.”
The philosophy of constant improvement is embedded in LTS’s thinking across product design, development and manufacture. This was the ultimate driving force behind the company’s ambition to enhance the production process for the on-body injector (OBI) configuration of the Sorrel™ platform. LTS believes that the introduction of greater levels of automation into a manually driven process can facilitate increases in capacity, process quality and resilience while reducing downtime and waste.
THE SORREL™ PLATFORM
The Sorrel™ device occupies a valuable space in the on-body delivery system landscape. The beating heart of the platform is the software-controlled electromechanical pumping system, which can be programmed with the desired infusion profile. This is true of both the recently commercialised point-of-care loaded OBI, as well as the vial and container compatible, prefilled and preloaded 5–20+ mL device, currently in development with multiple pharmaceutical partners (Figure 1).

Figure 1: The LTS Sorrel™ platform device can be converted to accept 5–20+ mL vials or cartridges with a simple volume adapter.
The platform’s underlying technology allows the device to deliver multiple benefits for both patients and pharmaceutical partners alike. For patients, and particularly those requiring frequent dosing of injectable therapies to manage long-term conditions, Sorrel can provide automated drug delivery of high-volume and viscous therapies via the convenience of a wearable device. Coming preloaded and prefilled, the device is intuitive to use, with no requirements for the patient to perform additional interim steps while administering an injection. Sorrel™ also encourages patient confidence in the process through the incorporation of audio and visual indicators to signify injection success.
For pharmaceutical partners, the benefits of the Sorrel platform are multitudinous. The fact that the device can incorporate standard vials as well as cartridges simplifies and accelerates the development process, while also maximising compatibility with established filling processes. Additionally, the potential to configure Sorrel for proprietary cartridges via minor adjustments to its outer casing offers additional flexibility. Whether using vials or cartridges, Sorrel simplifies loading by including a disinfection chamber with an integrated UV-C LED, which prepares the primary container’s septum before engagement with the device’s fluid path.
OPTIMISED PRODUCT DESIGN AND PRODUCTION
These well-established benefits are built on the strengths of Sorrel’s optimised product design, and further opportunities for optimisation have been identified in its manufacture. In theory, introducing greater levels of automation into the device’s manually driven production processes would secure increases in capacity, process quality and resilience while reducing downtime and waste. These valuable outcomes support the forward momentum within the LTS Device Technologies business, as exemplified by the previously referenced development projects and the 2024 commercialisation of the Sorrel OBI device.
“LTS’s flexible change-management strategies ensured project continuity in the face of any potential operational or logistical challenges.”
Despite deciding on a move to semi-automated production, LTS’s intention was to ensure that the design, assembly methodology and process controls for the Sorrel device remained consistent throughout the transition. In view of this, the challenge centred on maintaining the current assembly methodology while designing and implementing automation-based process improvements that would result in the anticipated production gains. More specifically, this meant targeting a production capacity of at least one part per 40 seconds and, in terms of quality, introducing better traceability for rejected non-conforming subassemblies/parts, as well as bringing in automated decision making for inspection procedures. At the same time, LTS’s flexible change-management strategies ensured project continuity in the face of any potential operational or logistical challenges.

Figure 2: This project underscores the need for seamless integration of highly qualified technicians and automated processes.
THE TRANSITION TO SEMI-AUTOMATED PRODUCTION
At a strategic level, the move from manual-based operation to semi-automated operation was pursued for several reasons, including the ability to achieve higher throughput because of extended periods of continuous operation. In addition, automation allows for complex operations to be accelerated and tasks to be performed at a level that reliably delivers a high degree of accuracy and consistency. Operatives continue to be integral to the process but, supported by automation, weaknesses associated with human error are reduced and output is enhanced (Figure 2).
Having identified the opportunity to realise process benefits – and therefore business benefits – by moving to a semi-automated production line, the next phase involved defining these targets in more detail. This encompassed setting objectives for production capacity, as well as listing expected improvements to existing processes and process controls.
Once established, these objectives were translated into a series of technical requirements that formed the process specification for LTS’s sub-contracting partner, teamtechnik, which was then tasked with implementing the semi-automated production line. The next phase of the project encompassed system design, build and installation, which incorporated factory acceptance testing and then, later, site acceptance testing at LTS to validate the installation and configuration of the machinery according to the previously defined specification.
Following the successful completion of these assessments, LTS was able to conduct pilot tests to assess the performance and reliability of the semi-automated systems in a run-up phase. This period was essential for troubleshooting any post-installation issues that had arisen, while also allowing any further opportunities for process improvement to be identified prior to full-scale implementation. Finally, the transition to the semi-automated production line was confirmed via process validation, with completion of all associated documentation (Figure 3).
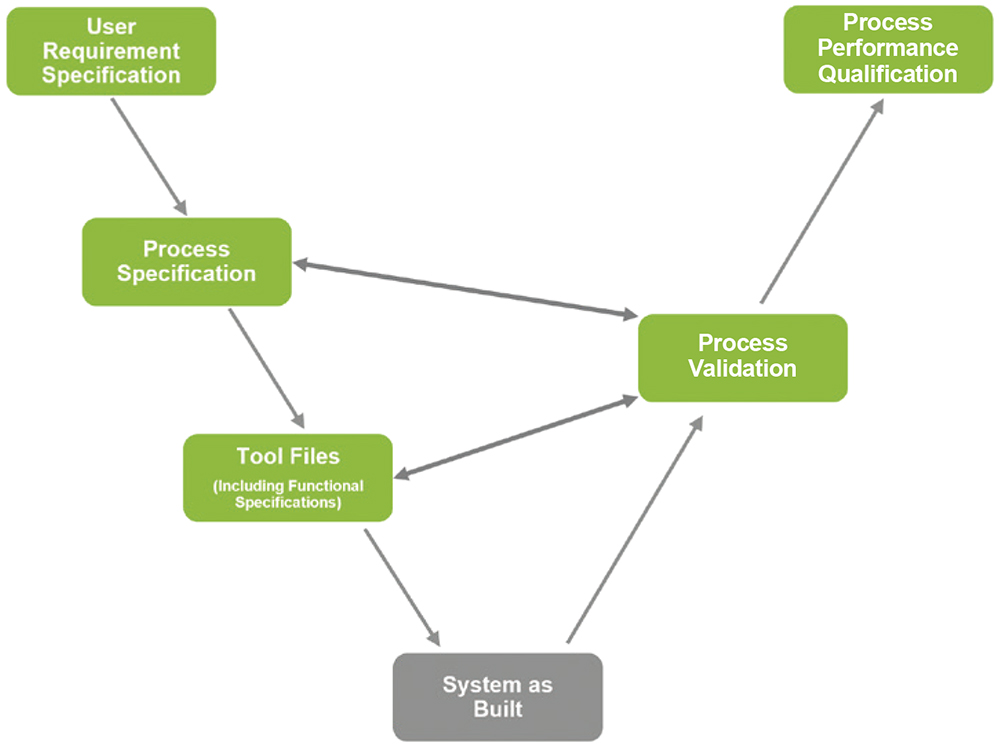
Figure 3: Project process overview.
SYSTEM IMPLEMENTATION
As expected with any process driven by continuous improvement, the implementation of the system was regarded as only the beginning of a new phase, ensuring that it continues to benefit from ongoing optimisation. A key challenge in this phase is the identification and definition of required maintenance procedures, with a view to limiting unnecessary downtime and controlling any planned interventions.
In such a complex system, containing a multitude of moving parts, maintenance is inherently complex. There are interdependencies between components to consider, making it essential to take a holistic view of the entire production line to avoid making any changes that trigger an unwanted domino effect. Furthermore, the task of creating accurate, detailed documentation covering all aspects of such a system becomes far more extensive and onerous. In addition, it is critical to consider the availability of replacement parts, adherence to safety procedures during maintenance and cleaning operations, and integration with computerised maintenance management systems to streamline scheduling, tracking and reporting.
Figure 4: Speed of innovation – technicians interacting with automated equipment.
And, while integrating the new system with IT processes is crucial to operational success, this also depends heavily on successful integration with the workforce tasked with controlling and managing the system on a daily basis, as well as supporting its development over time. At the design stage, this means thinking about the direct interface between the two, with the ergonomic needs of the workforce incorporated into the resulting equipment installation (Figure 4).
Building the team’s capabilities was also a crucial part of the transition from manual to semi-automated production. Employees were given comprehensive training on the system itself, providing them with a thorough grounding on the technology. This was supplemented by continuous learning opportunities to ensure that they were kept up to date on any technology and process developments. In parallel, engineering specialists were provided with the knowledge to troubleshoot issues and support operational continuity.
PROCESS VALIDATION
In the final stages of implementation, a comprehensive process-validation programme had to be carried out and documented. Completing this step is not only a regulatory requirement but also ensures that all aspects of the system comply with expected standards, and that the processes combine to produce products that meet the defined specifications reliably.
In medical device manufacturing, guidance on process validation for quality management systems is provided by the Global Harmonization Task Force. This documentation has supported manufacturers in carrying out process validations for more than two decades, and provided LTS with an authoritative reference for all key activities that are encompassed within this critical stage. As per the guidance, the overall intention is to verify that, taken as a whole, the production process “has been subject to such scrutiny that the result of the process can be practically guaranteed” and that when operated within specified limits it “will consistently produce product complying with predetermined requirements”. Process validation covers three main phases (Figure 5):
- Installation qualification, which demonstrates that the equipment has been correctly installed and calibrated according to the manufacturer’s specifications.
- Operational qualification, which demonstrates that the process produces acceptable results and allows parameter limits to be set.
- Performance qualification, which demonstrates long-term process stability under the strain of a large manufacturing load.
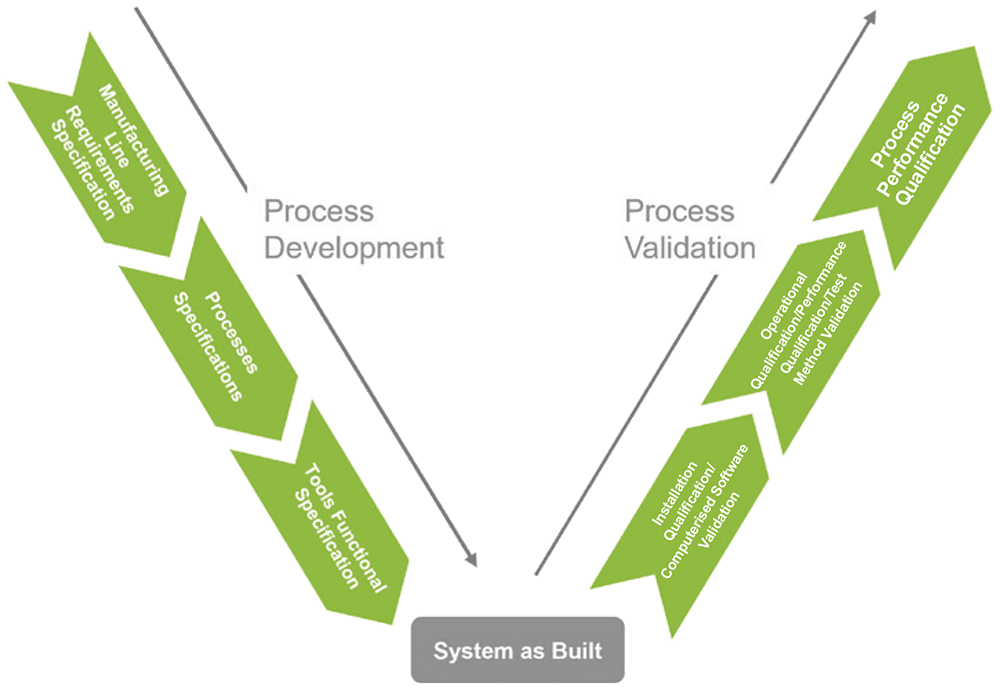
Figure 5: Overview of the process development and validation processes.
These priorities were all incorporated into a master validation plan along with processes for computerised software validation and validation of testing methods. If any of these validation processes generated data outside of the target range, corresponding updates were made to the process and functional specification to correct the situation. Further validation testing would then be conducted until this feedback loop was closed. Finally, the system was subject to process performance qualification to demonstrate its capability in consistently outputting products that meet the quality specifications.
The extent of the entire process validation stage, which took only ten months to complete, highlights the delicate nature of adopting change in pharmaceutical manufacturing environments. In this instance, LTS’s ambition was not to change the existing assembly methodology or in-process controls, yet still the project represented a significant undertaking.
“Through strong collaboration and a process-driven approach, the achievement of transitioning from a manual to a semiautomated production line has delivered important benefits in line with LTS’s initial target objectives.”
CONCLUSION
Through strong collaboration and a process-driven approach, the achievement of transitioning from a manual to a semiautomated production line has delivered important benefits in line with LTS’s initial target objectives, including enhanced quality of output, improved consistency in production and the ability to produce a device every minute. LTS has also been able to identify potential future changes that could double or possibly triple its productivity, and these will be implemented over the coming months and years as the company continues on a path of continual improvement.
As it stands today, however, LTS is now equipped with a fundamentally optimised production-line blueprint that is ready for global deployment. This builds neatly on the synergy that has already arisen from the LTS Lohmann acquisition of Sorrel™ in terms of positioning the device as a truly international device platform. Indeed, a project is already underway to establish worldwide manufacturing capability for Sorrel via multiple geographical production locations.
Change is often regarded as an unsettling force but, by trusting in continual improvement and pursuing a carefully implemented change-management process, LTS has been able to realise manufacturing enhancements that will not only benefit its existing and prospective international partners but also individual patients across the world who rely on wearable devices to alleviate the pressure of managing chronic conditions.

