Citation: Monroe N, “Connectivity Restoring Trust in Pharma Communications”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 12-16
Napoleon Monroe analyses connectivity coverage at recent drug delivery conferences and, more broadly, in various publications, to evaluate the current direction, and explore the scope for connectivity to effect changes benefiting multiple stakeholders across a range of treatment categories.
“Connectivity can help communicate value to, and help build relationships and instil trust with, patients.”
The drug delivery collaboration conference season peaks with three conferences – Partnership Opportunities in Drug Delivery (PODD) in Boston, MA, US (October 7-8, 2019), PDA’s Universe of Pre-Filled Syringes and Injection Devices (PDA) in Gothenburg, Sweden (October 22-23, 2019) and Drug Delivery Partnerships (DDP) in Orlando, FL, US (March 30-April 1, 2020).
ONdrugDelivery is a media sponsor for all three, and other related conferences, and often presents highlights of products and trends arising at these events in its publications. It works the other way around too. For example, my February 2019 ONdrugDelivery Expert View, “Connectivity Using Consumer Technology to Create Real Value for Patients”1 was distributed at Drug Delivery Partnerships in Palm Beach Gardens, FL, US, the same month. Our presentation at DDP was based largely on the article, and so the flow of useful information, insights, and ideas can flow both ways.
At a pre-conference DDP 2019 presenters’ dinner, I asked which companies were engaged in connectivity. The response was clear: “Who isn’t?” Several highly interesting observations important to connected drug delivery came out of the DDP meeting, and a lot of relevant news has been published since. This article will review some highlights.
One broad observation is about the themes of conference presentations, as shown by keywords in the titles: partnerships, progress, evaluation, sensors, innovation, perspective, treatment, development, commercialisation, marriage, choice, design, requirements, outcomes, evolving, challenges, centricity, approval, management, training, onboarding, value, compliance, adherence and connectivity. All of these words appeared in event presentation titles and ALL are integrally related to communication. To be effective, communications must be trusted.
During the DDP 2019 Q&A session in the “Creating Value in Your Patient Centric Platform” panel, Paul Jansen of Haselmeier noted that “Pharma has lost the trust of the patient community as a result of recent pricing policies, especially as it relates to epinephrine”. Pricing was, and is, only one key issue, and epinephrine just one example where trust has been impacted by pricing policies. Yes, the EpiPen, a very visible recent poster child for reputationally destructive pharma behaviour, was called “infamous” by a presenter at PDA 2018, and is cited in a March 2019 Pharmaceutical Manufacturing piece, “Pharma’s Damaged Reputation”.2 That article discusses some reasons why pharma “has not developed better strategies around ethics and innovation”. After DDP, came news that the Pfizer plant which makes the EpiPen is under US federal investigation for quality issues and the news of drug price fixing suits by some >40 states.
Please allow me to note that my colleagues and I were proud of the work we did in the 1970s and 1980s on the nerve agent antidote autoinjectors and developing the original EpiPen, both life-saving delivery systems. The ethics of the time and our corporate management allowed us to focus on the products, not the quarterly financials. However, in my opinion, thus far pharma has not concentrated effort of the widely discussed value to patients. Note that the American Association of Retired Persons (AARP), for example, has 38,000,000 members all aged 50+, so their members are in the age group for whom the most pharma is purchased. The AARP’s May 2019 Bulletin featured an eight-page cover story entitled “A 5-Point Plan to Lower Prescription Drug Prices”, which slammed drug pricing.3 In face of this, how is pharma to recover trust?
Connectivity can help communicate value to, and help build relationships and instil trust with, patients. The loss of trust is far broader than with the pharma industry, but here a focus will be using connectivity in the pharma industry to build trust.
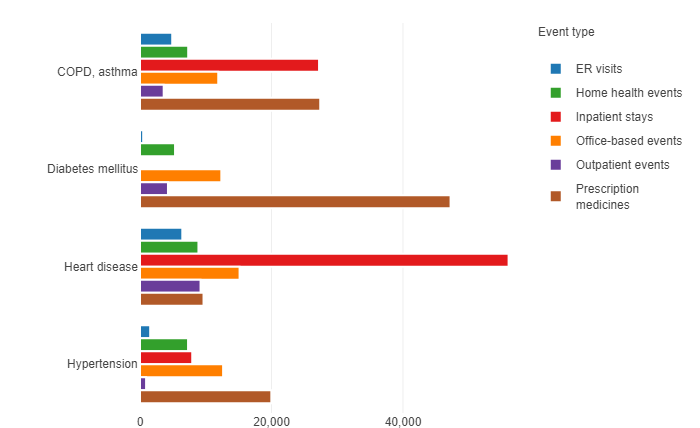
Figure 1: Total US expenditure (US$ million) by condition and event type, from the 2014 Medical Expenditures Panel Survey of the US DHHS Agency for Healthcare Research and Quality.
In conversations at DDP, a couple of respected colleagues stated that diabetes is different from all other combination product disease treatment markets as regards connectivity. They opined that the only disease category that made sense for connectivity was diabetes. I agree in so far as diabetes is the only category where connectivity has already achieved broad patient and profitable pharma success.There are good reasons why diabetes is where treatment success and profitability have already been achieved. But that is not to say that the applicability of those reasons begins and ends with diabetes.
While the case for diabetes connectivity is compelling, what has been achieved in past certainly does not represent the limit of future achievements for connectivity. Further, the case for diabetes connectivity does not negate or speak to the possibilities for improving treatment in almost all other disease categories.
The characteristics of diabetes treatment that have allowed connectivity to have already been successfully added to combination products are rarely unique to the diabetes market. Please consider all of the following characteristics shared with other therapeutic areas and treatment categories:
- The large size of the global market
- The mode of administration of insulin is traditionally and most commonly injection. Ongoing R&D in IoT and pens for insulin injection has advanced many designs
- Well-established drug treatment classes for diabetes
- Availability of diagnostic means, also connected diagnostic products
- Patients can often feel the onset of physiological change requiring treatment
- Recognition that failure to treat is a real threat
- Frequency, complexity and variability of the treatment
- Potential for debilitating effects of the disease (loss of mobility and eyesight) from non-adherence / non-compliance
- Potential for life-threatening emergencies (e.g. diabetic ketoacidosis)
- Diabetes triggers many emotions
- Concentration of leadership in a few large companies and their partners who are competing for commercial advantage for their expensive and profitable products and working to lead in and improve their positions in the category.
Expanding on the last point, companies with connected diabetes combination products include: AstraZeneca, Novo Nordisk, Eli Lilly, Roche/mySugr, Dexcom, Abbott, Glooko, and Companion Medical. These companies have secured management support and approvals for large expenditures for connectivity. Some have FDA approvals, many more approvals are pending. The companies believe they have, or can secure, all necessary regulatory approvals and freedom to operate at least in the US. All need to fend off pricing criticisms.
Creative approaches to diabetes treatment connectivity continue to evolve. At DDP, co-operations among Haselmeier, CommonSensing and Flex for a cap for insulin pens and other injection devices was announced. CommonSensing is going forward with clinical studies.4
EXPENDITURES BY DISEASE
Data from the 2014 Medical Expenditure Panel Survey (MEPS) from the US Dept of Health & Human Services’ Agency for Healthcare Research and Quality, by service for diabetes, coronary and pulmonary disease are quite interesting and are likely to still be accurate in relative terms (Figure 1).
Let’s look at the expenditure for diabetes as compared with COPD and asthma. Note that none of the three diseases is curable. Asthma, like diabetes, has a significant paediatric population. Once diagnosed, patients will likely continue some therapy for life.
There are many longstanding tools providing connectivity for diabetes. Major pharma leaders and their partners in inhaler treatments for asthma and COPD have only recently announced connected inhalers. Companies with programmes for connected inhaler combination products include: AstraZeneca, GSK, Merck & Co, TEVA, Sumitomo (Sunovion), Biocorp, H&T Presspart, 3M, Propeller Health, Adherium and others. Like those with connected diabetes programmes, these companies have secured management support and approvals for large expenditures for connectivity in the area of inhalation, have some FDA approvals with many approvals pending. All need to fend off pricing criticism.
The discrepancies between the figures for diabetes expenditures and those for the two pulmonary diseases are rather fascinating. They may be partly related to the many more years of work on the development of connectivity to improve the effectiveness of diabetes care versus the relatively recent innovations in asthma and COPD.
Analysis
The cost of inpatient stays directly for diabetes are minimal. Proper training and self-treatment can significantly reduce incidence of diabetic retinopathy, diabetic neuropathy and diabetes associated vascular disease.5 Blindness and amputations of lower extremities are dramatic, but other co-morbidities related to untreated diabetes of diabetes also benefit from patient training and other assistance with adherence. Connectivity represents a tool for such training and assistance.
| Broad Treatment Category | Examples / Drug Categories |
| Treatments subject to abuse | Opioids and other pain management drugs |
| Emergency, potentially life-saving, rescue medications | Anaphylaxis, asthma, COPD, opioid overdose reversal, acute hyperkalaemia, acute hypoglycaemia, nerve agent antidotes, organophosphate and other insecticide poisoning antidotes |
| Life-sustaining medication | Diabetes, multiple sclerosis, epilepsy (both human and veterinary), ALS, nonalcoholic steatohepatitis (NASH) (fatty liver disease) treatments, antibiotics and other post-surgical drugs, autoimmune disorders including endocrine deficiencies such as Addison’s, haemophilia, drugs to avoid pre-term delivery, cardiac disease management. |
| Treat to cure | Hepatitis C, tuberculosis, ebola, osteoporosis, some cancers |
| Enhance quality of life | Sexual dysfunction, growth deficiencies |
| Control disease severity and prolong life |
Cancer, AIDS treatments, psychotropic drugs |
| Disease avoidance | Vaccines, antibiotics, antivirals, antifungals |
| Predictors of disease progression, exacerbations or other severe conditions |
Benadryl or rescue inhaler over-use as a predictor of worsening allergic issues |
| Adjunctive pharma to enhance or prolong the effectiveness of non-drug therapies |
Post-surgical antibiotics, post-transplant immunosuppressants |
| Control or avoidance of symptoms | Plaque psoriasis, migraine, pain, severe behavioural or psychological dysfunction (schizophrenia being among the most severe), adrenal deficiency diseases such as Addison’s |
| Emergency kits containing drugs for use by untrained individuals | Anaphylaxis |
| Office-based diagnostic and treatments not currently easily controlled or recorded | Some sequence- and time-sensitive medical and dental products |
| Veterinary products in some of the same treatment categories | Canine seizure |
Two comparisons are most instructive: the US cost of inpatient stays for asthma and COPD was some US$27 trillion as of 2014 and growing. Inpatient stays directly for treatment of diabetes did not register on the charts; and Emergency Room visits for diabetes were minimal compared with those for COPD and asthma.
“Having experience with such a product in the hands
of many patients should provide a wealth of human factors information. This is in line with FDA’s stated objective of securing more rapid approvals and
real-world data to provide real world evidence.”
As covered in my 2019 article for Inhalation6, and in other articles in ONdrugDelivery, administration by inhalation requires adherence to a difficult technique, reactions may vary by patient, and inhalation therapy can benefit from connectivity.
Whilst US prevalence of asthma in 2016 was estimated to be 26,000,000 diagnosed7 a large number of individuals, especially children, are undiagnosed. And in COPD, 2010 prevalence was 15,000,000 diagnosed with an estimated 12,000,000 potential cases remaining undiagnosed. For diabetes, 2017 estimates were that 23,000,000 cases were diagnosed with 7,200,000 undiagnosed.8 Almost twice as many deaths are attributed to COPD as are attributed to diabetes.9
The high cost of hospitalisations and ER visits for the other diseases include in the MEPS demonstrates opportunities for improvement. These diseases have similarities with diabetes, such as large markets, recognition that failure to treat is a real threat, potential for debilitating effects, potential for life-threatening emergencies, patients can sometimes feel the onset of physiological change requiring treatment. For asthma and COPD, the fact that patients can often feel the onset of physiological change requiring treatment.
In cardiology, the emerging availability of diagnostic means is important. Heart disease and hypertension are often “silent” diseases until they demand hospitalisation. Patient-use diagnostics for coronary diseases are only recently becoming available with the approvals of AliveCor’s Kardia Mobile and the Apple Watch, which has the ability to diagnose the onset of certain abnormal cardiac events. These open possibilities for remote diagnostics for atrial fibrillation and other cardiac anomalies may enable future opportunities for combination product connectivity and cardiac self-treatment. A patient-use stroke diagnosis classification (haemorrhagic versus thrombolytic) would be a real breakthrough. Additional research is underway and cardiac self-treatment is likely to be enabled by the approvals of wearable diagnostic devices.
It is clear that connectivity can help meet stakeholder needs. The benefits are often different for different products and for different stakeholders. For example, patient needs are served by making self-treatment possible, making convenient for them to treat themselves, and limiting costs. Medical provider needs are served by monitoring use in order to understand patient response, safety and efficacy. Regulatory expectations for real-world evidence can be met. These have become more important and widespread. Payor desires are served by enabling pay-for-performance models. Societal needs are met for preventing abuse. For all of these stakeholders, connectivity offers solutions.
Table 1 lists numerous disease categories for which connectivity has been explored. Many of the categories have already seen significant investments (asthma and COPD now have connected products approved, for example).
Some listed in the table fit in a super-broad category where connectivity has potential including, for example, expensive treatments for any condition (in particular severe conditions) where compliance is essential to rescue, cure, management, remission or avoidance. With a regulatory acquiescence (regulatory discretion), products falling into this category, providing information to the patient, could be in line with the “C-CONTAINER” approach described in “Connectivity Using Consumer Technology to Create Real Value for Patients”.1 The approach would necessarily limit claims made – companies could enjoy the advantages of early connected product introduction with limited regulatory delay. Having experience with such a product in the hands of many patients should provide a wealth of human factors information. This is in line with FDA’s stated objective of securing more rapid approvals and real-world data to provide real world evidence.
BEYOND WHAT’S COVERED AT CONFERENCES
In addition to reviewing the hot topics at drug delivery conferences, it instructive to observe what is not covered. There was little or no mention of the US Drug Supply Chain Security Act or electronic medical records (EMRs) at either PDA or DDP. My “Connectivity Using Consumer Technology to Create Real Value for Patients”1 article, for example, pointed out that there were problems with existing EMRs and that there was a need to make more effort to benefit from public initiatives.
After DDP, a Fortune article entitled “Death by a Thousand Clicks”10 stated that “Ten years and 36 billion dollars later the EMR system is an unholy mess”. Soon after that, in an interview with Time Magazine,11 Eric Topol said: “The biggest problem in medicine is that we use keyboards and screens and it’s led to the depersonalisation of the doctor-patient relationship.” His views echo those of many other practitioners. His proposed solution is to “have patient data assimilated, slides and scans read and analysed. That liberates doctors from keyboards.”
The HHS Office of the National Co-ordinator for Health Information Technology (ONC) recently issued a press release covering guidance on interoperability, transparency, patient access and restrictions on information blocking. If these rules are implemented, they would drive a sea change in how healthcare information systems are used.12 The same day, the Trump administration signed an Executive Order on maintaining US leadership in artificial intelligence.13
CONCLUSION
I’m hopeful that in the pharmaceutical industry, pharma companies and their partners can overcome the challenges and take the lead in improving communication, building trust, and improving outcomesby making more and better use of connectivity across the important treatment categories highlighted here.
Biotech and other specialty products are generally expensive to manufacture, command high prices, are profitable and sensitive to environmental conditions. The importance of the biotech sector to pharma and patients has driven the development of new delivery devices (combination products) to enable patient home use. Home use is not limited to specialty, but specialty is where the money and in the case of injectables, where the most challenging human factors problems, are. These facts and others discussed here are crying out for connectivity to take an increasing role.
The possibilities for connectivity to bring improvements for all stakeholders are immense and, even though the work has begun, the opportunities connectivity presents that remain largely untapped are vast, to say the least.
REFERENCES
- Monroe N, “Expert View: Connectivity Using Consumer Technology to Create Real Value for Patients”. ONdrugDelivery, February 2019, Issue 95, pp 11-15.
- Parrish M, “Pharma’s Damaged Reputation”. Pharmaceutical Manufacturing, March 18, 2019.
- Marsa L, “A 5-Point Plan to Lower Prescription Drug Prices”. Am Assoc Retired Persons Bulletin, April 30, 2019.
- Munshi M et al, “Nonadherence to Insulin Therapy Detected by Bluetooth-Enabled Pen Cap Is Associated With Poor Glycemic Control”. Diabetes Care, 2019,Vol 42(6), pp 1129-1131.
- “Can diabetes cause blindness and amputations?”. Diabetes FAQs Section, Health 24 Web Page, November 4th, 2014.
- Monroe N, “Connected inhalers: Stakeholders’ potential benefits and possible concerns”. Inhalation, October 2018.
- “Most Recent National Asthma Data”. US Centers for Disease Control & Prevention Web Page. (www.cdc.gov/asthma/most_recent_national_asthma_data.htm, Accessed May 2019.)
- “National Diabetes Statistics Report”. Research Report, US Centers for Disease Control & Prevention, 2017.
- “Leading Causes of Death”. US Centers for Disease Control & Prevention Web Page. (www.cdc.gov/nchs/fastats/leading-causes-of-death.htm, Accessed May 2019).
- Fry E, Schulte F, “Death by a Thousand Clicks: Where Electronic Health Records Went Wrong”. Fortune Magazine, March 18, 2019.
- Park A, “Cardiologist Eric Topol on How AI Can Bring HumanityBack to Medicine”. Interview,Time Magazine, March 14, 2019.
- “HHS Proposes New Rules to Improve the Interoperability of Electronic Health Information”. Press Release, US Dept of Health & Human Services, February 11, 2019.
- Trump D “Executive Order on Maintaining American Leadership in Artificial Intelligence”. Executive Order, US White House, February 11, 2019.
Previous article
DON’T DEVELOP A CONNECTED DRUG DELIVERY DEVICE WITHOUT READING THISNext article
MIRCEA DESPA & DOUG McCLURE, BD

