Citation: Williams N, “Low-Cost Connectivity Meets Patient & Pharma Medication Management Needs”. ONdrugDelivery Magazine, Issue 98 (Jun 2019), pp 38-41.
Neil Williams describes the potential connected drug delivery systems hold to address serious challenges in healthcare, including poor adherence, and also deliver substantial value, and outlines the company’s highly scalable enterprise platform, the Connected Health Platform (CHP).
A digital revolution is transforming healthcare across the globe, and it’s high time drug manufacturers got in on the action. This new landscape runs the gamut from continuous glucose monitoring (CGM) and remote patient surveillance, to point-of-care test results in the clinic and at home. Artificial intelligence (AI) is establishing virtual diagnosis and care pathway plans, while e-prescribing systems are informing clinicians about drug compatibility and formulary preferences. However, digital data related to medicine adherence is conspicuously lacking. Without accurate, detailed knowledge of when – or even if – patients take their prescribed medication, it is nearly impossible to gauge the effectiveness of drug therapies or to manage diseases.
“Our estimates show that a 3% increase in adherence has the potential to generate an additional $1-3 billion in revenue over five years, far offsetting the cost of investment in connectivity.”
Monitoring, measuring and supporting patient adherence to prescribed medications is vital, especially in today’s value-based healthcare environment where outcomes are the new income for patients, providers, payers and pharma. Poor adherence rates take a toll in terms of reduced patient health and quality of life, as well as financially. An estimated US$290 billion (£229 billion) in avoidable US healthcare costs – approximately 13% of the total – has been attributed to poor medication adherence.1
Pharmaceutical companies also pay a high price for these low adherence rates, which account for approximately $188 billion in lost revenue.2 With adherence rates of 31-66%, common auto-immune, diabetes and respiratory medications leave an estimated $1-10 billion dollars “on the table” annually.2
Yet our estimates show that a 3% increase in adherence has the potential to generate an additional $1-3 billion in revenue over five years, far offsetting the cost of investment in connectivity.
UNDERSTANDING THE CHALLENGES
Connected health solutions for medication management, patient support and analytics offer promising potential for addressing the unique medicine-related needs and challenges faced by key stakeholder groups in healthcare, including patients, clinical researchers/data scientists, pharmaceutical manufacturers, and families and friends.
Patients
The ability to track their medication dosage, frequency and health via a mobile app, to receive alerts and reminders, and to access helpful information about their condition, helps patients maximise their medication benefit and manage their disease or chronic condition more successfully. When patients use a connected drug delivery device such as an inhaler or injector with a regulated Mobile Medication Application (SaMD/MMA), they can easily and accurately capture data about when they take their medication and how much, right on their phone, tablet or other smart device.
The number of health apps is extensive, but they certainly don’t all offer equal value. More than half of the apps on the market perform only a single function, such as inform, instruct or record data.3 A mere 6% feature medication reminders.3 Without the ability to remind, alert, communicate or share data, apps serve as little more than digital diaries. To engage patients effectively, apps should feature a human factors-tested, patient-centric design, and potentially include motivational tools that are cohort based to encourage adherence and lifestyle choices.
“Without the ability to remind, alert, communicate or share data, apps serve as little more than digital diaries. To engage patients effectively, apps should feature a human factors-tested, patient-centric design, and potentially include motivational tools that are cohort based to encourage adherence and lifestyle choices.”
Connected health solutions built on Phillips-Medisize’s Connected Health Platform (CHP), for example, can be integrated with a highly secure, regulated mobile app platform (Figure 1) that provides medication scheduling and reminders, adherence tracking and contextualised educational content tailored to individual patient needs. The app can also integrate patient-reported outcome measures (PROM), data from other monitoring devices the patient may use at home as well as external data such as hospital lab results, to provide a more holistic view of the patient’s condition in real-time.
Providers
Typically, healthcare specialists see patients with chronic conditions only once or twice a year, unless the patient’s risk profile deteriorates. In those cases, it may be too late for the provider to have meaningful impact on the patient’s health. However, the ability to monitor data from a connected drug delivery device, integrated with other patient data, provides an opportunity to work with observed rather than expected results and stratify patients by risk so that health professionals can swiftly identify patients at risk of significant deterioration and therefore intervene.

Figure 1: The Connected Health Platform can be integrated with a highly secure, regulated mobile app platform for patients.
By using a connected health platform’s analytics component to risk-stratify those patients, care providers can intervene on a timely basis in a number of ways, such as calling, messaging or requesting the patient schedule a clinic visit. In addition, AI and content management capabilities can be used to help patients better manage their health. For example, clinicians can send a message reminding the patient, “You’ve been missing your evening injection. Here’s why it’s important to have it.” They can also include multimedia educational content targeted at the patient.
Phillips-Medisize’s platform can create healthcare practitioner (HCP) dashboards (Figure 2) and alerting tools, and integrate patient data directly into a clinical portal and the healthcare system’s electronic health record (EHR) system or to a regional health information exchange (HIE). The bidirectional capability allows patients to send their drug delivery data to the hospital but also to receive medical images, lab results, care continuity information from their providers and other data, so they can access and own their medical data.
Clinical researchers/data scientists
The Phillips-Medisize CHP allows data scientists to create new dashboards and conduct analytics that support their research on the effectiveness of various drug therapies, and all data within the platform is compliant with US FDA CFR 820 Part 11 standards. Data on adherence and effectiveness can also be shared with payers, if patients give permission and the pharmaceutical company is willing.
Pharma Manufacturers
Connected health solutions offer value to pharma companies both during the early-stage clinical trials of a new drug as well as in Phase IV studies once the drug is commercialised and on the market with Part 11 compliant data. By differentiating and improving the drug delivery and digital experience, and identifying patient cohorts, more prescriptions can be written, in turn improving patient engagement and generating additional revenue.
Access to connected health solution data also has the power to radically transform patient support programmes (PSPs), which play key roles in furthering adherence. Current PSPs run by pharma companies take a proactive approach to supporting patients in their homes or other remote care settings in order to promote better compliance and improved outcomes. These programmes often provide brochures, letters, phone calls, messages, targeted video content and emails. Many also have patient contact information and patient/condition guidelines. But until recently, they have not had accurate, timely information about patient health on a regular or on-demand basis.
A connected health solution allows physicians, nurses and other patient support professionals to view how patients are doing, enabling them to devote more time and energy to those who need greater support. They then can tailor patient care based on real-time feedback, with potentially significant impact.
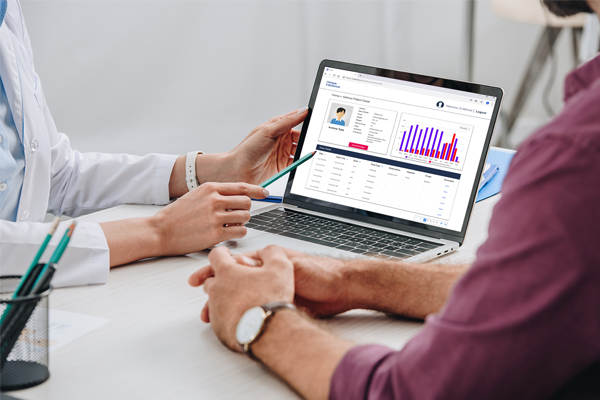
Figure 2: Healthcare practitioner dashboards and alerting tools integrate patient data directly into a clinical portal and the healthcare system’s electronic health record system or to a regional health information exchange.
Families & Friends
In addition, it enables patients to connect with other patients via a closed or public social media platform. Sharing similar challenges, experiences and insights can be valuable in supporting adherence in an age when nearly one-quarter of consumers say they use social media to get information about health options.4 Phillips-Medisize’s CHP supports integration with social media platforms and also includes a discrete social community capability.Connected health solutions provide an opportunity for families, friends and caregivers to monitor via a mobile app how their child, elderly parent or other family member is doing so they can offer help as needed. After all, sometimes mothers, fathers, sons, daughters or other relatives or friends who have a trusting relationship with patients are more effective persuaders about medication adherence and healthy behaviours.
TAKING THE LEAP TOCONNECTED HEALTH
With the cost of new drug development pushing $2.6 billion,5 many pharma manufacturers are understandably wary of investing significant additional funds in connectivity. As a result, only a few connected health solutions related to drug delivery are on the market to date. But the upside for unlocking the value in connected health and digital intervention is sure to drive initiatives.
Three primary components comprise the connected health ecosystem: connected devices, such as inhalers and injectors; digital interfaces, including patient apps and HCP dashboards; and a cloud platform that enables data integration with multiple sources to generate insightful analytics (Figure 3). The opportunity to build drug delivery devices and healthcare apps on an existing connected health platform helps pharmaceutical companies, drug delivery device developers and medtech manufacturers substantially reduce risk, cost and time to market.
“Three primary components drive the cost of connectivity: integrated circuits, sensors and batteries.”
Since developing the first connected health combination product (including autoinjector, app and cloud platform), approved by the FDA for a specific drug four years ago, Phillips-Medisize has invested in creating a highly scalable enterprise platform for the evolving and expanding market.
Listed as a Medical Device Data System (MDDS), the Phillips-Medisize platform can be configured “out of the box” to support multiple therapeutic areas affording patients with different diseases and medications a targeted experience including multiple apps and associated device(s), if applicable. The forthcoming Phillips-Medisize healthcare app framework can be quickly customised to link patients and devices to the platform. These capabilities, combined with the other advantages Phillips-Medisize offers as an end-to-end solution provider of medical products and connected health systems, create value from strategy setting through to on-market drug delivery and digital engagement for pharma, device and medtech manufacturers seeking to capitalise on connected health solutions. The third-generation CHP offers several benefits including comprehensive information-sharing and analytics capabilities, robust cybersecurity, streamlined regulatory documentation, a modular approach, the capability to support global franchises with normalised clinical coding and post-market surveillance pharmacovigilance reporting within a per client “private cloud.”
During the strategy, feasibility and development phase, the Phillips-Medisize team works closely with the pharma company to create a patient-centered design that improves the drug delivery and digital intervention experience far more effectively – and efficiently – than an off-the-shelf solution where designs are already fixed. The existing regulatory documentation packs also mean a solution will be ready for a US Part 11 Compliant study, premarket submission for 510(k), Combination Product submission and EU CE mark filing sooner; helping to reduce project costs, regulatory approval documentation and, ultimately, time to commercialisation.
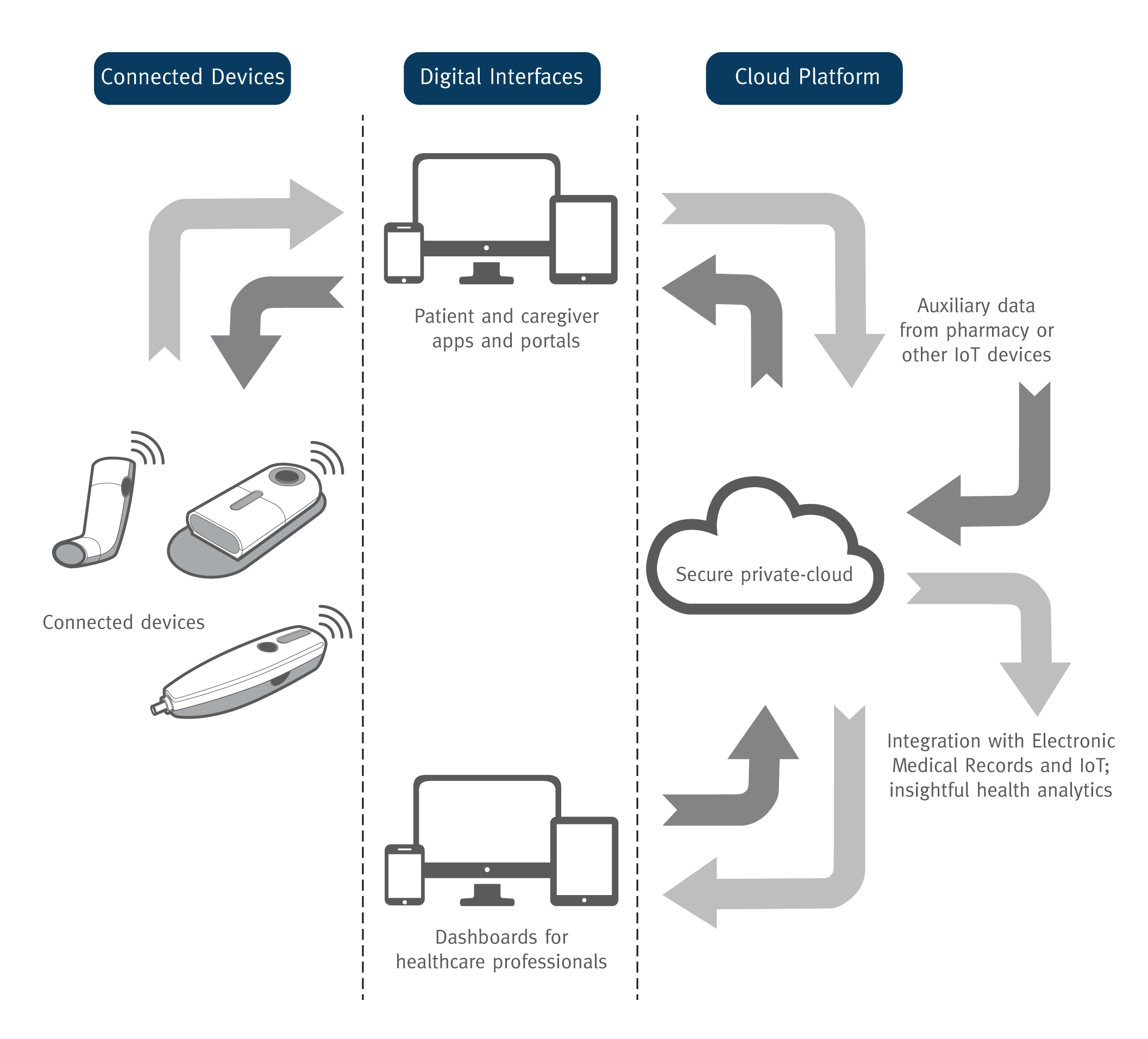
Figure 3: The three primary components of a connected health ecosystem.
The ability of Phillips-Medisize to manufacture the electronics internally also makes connectivity much more affordable, both for re-usable and disposable drug delivery devices. Three primary components drive the cost of connectivity: integrated circuits, sensors and batteries. As a Molex company, Phillips-Medisize can take advantage of its vast global electronics design, manufacturing capability and purchasing power to keep connectivity and total manufacturing costs – including the moulding, automated assembly, circuit board assemblies and metal stamping – low. In addition, all solutions are designed and manufactured to meet environmental standards.
Finally, Phillips-Medisize offers supply-chain integration as part of its end-to-end service. The company’s cold-chain storage facilities enable it to monitor and regulate the temperature of drugs delivered to load into the connected health solutions. These can then be shipped in climate-controlled containers directly to customers.
BUILDING VALUE THROUGH SMART DEVELOPMENT PRACTICES
Integrating connectivity into innovatively designed, patient-centric drug delivery devices provides promising potential for pharmaceutical companies and medtech manufacturers to better meet the needs of patients, healthcare providers, payers and clinical researchers. By improving the patient and provider experience, connected health solutions can help support increased adherence, potentially enabling greater compliance and persistence, as well as revenue opportunities from an engaged patient population.
REFERENCES
- Philipson T, “Non-Adherence in Health Care: are Patients or Policy Makers Ill-Informed?” Forbes Magazine, May 8, 2015.
- Moore T, Chawla S, Firlik K, “Estimated Annual Pharmaceutical Revenue Loss Due to Medication Non-Adherence”. Research Report, Capgemini and HealthPrize, November 2016 (updated version of 2012 edition).
- Aitken M, Lyle J, “Patient Adoption of mHealth: Use, Evidence and Remaining Barriers to Mainstream Acceptance”. Research Report, IMS Institute for Healthcare Informatics, September 2015.
- Atlur V et al, “Unlocking digital health: Opportunities for the mobile value chain”. Research Report, McKinsey & Company, April 2015.
- Sullivan T, “A Tough Road: Cost to Develop One New Drug is $2.6 Billion; Approval Rate for Drugs Entering Clinical Development is Less Than 12%”. Policy & Medicine, March 21, 2019.

