Citation: Fink D, Gaul S, “Assessing Usability Early for Optimal Injection Pen Design”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 82-85.
David Fink and Stefan Gaul use the example of Haselmeier’s D-Flex system to explain why it is important to undertake a full use-related risk analysis for injection pen products to assess their usability and identify any risks, highlighting the importance of considering usability right from the start.
Mistakes in the dose or method of using a drug were recognised almost two decades ago as significant errors in patient treatment, carrying serious consequences.1 Adverse drug events (ADEs) may number in the millions across the US alone in care settings ranging from hospitals to long-term care facilities.2 Adding to the milieu is the growth in self-injection, with effective treatment dependent on patients understanding the correct application process.
To address this risk, the rise in human-centred engineering practices in medical device development, combination drug delivery and associated treatments is acknowledged to be a key factor in improving treatment safety and efficacy. Regulatory bodies are leading that process, tying approval to effective application of human factors and usability engineering (HF&UE) practices through guidance documents3-6 for both the product and its associated packaging and labelling.
One element of this guidance is the application of use related risk analysis for effective product design and clear presentation (e.g. labelling, packaging and instructions for use) to the defined end users. Undertaking this analysis requires an understanding of the intended user, use environment and use flow of the selected device, and assessing the risk of anticipated errors that will guide potential solutions to mitigate those risks.
To understand how this process works, we will focus on a novel injection device design (Haselmeier D-Flex) that presents a flexible injection platform ideally suited to targeted therapies where HF&UE and consideration of potential usability issues were applied from the inception of the concept.
“Fixed-dose pens are limiting, while variable-dose pen injectors have such a broad array of dose choices that this may create confusion and errors. Haselmeier saw this as an opportunity to strike a new path.”
DEVELOPING A USE-CASE APPROACH
Haselmeier, with a long history of developing and producing injection pen devices, viewed emerging market trends as an opportunity to identify unmet or hidden needs which could be addressed in terms of improving usability and reducing injection errors.
Haselmeier’s strategic product management team investigated the market to identify indications of strategic relevance to be considered in the development of an innovative platform. This strategic consideration was matched with general trends such as changes in administration method, volume and viscosity, the frequency of administration, as well as comparisons with other devices available on the market. These insights comprised a basis to identify any promising business opportunities, resulting in a corresponding use case.
In order to design and develop an effective and valuable product or service that is desired in the market, the design team needs to consider the relevant use cases to know what needs to be developed. The use case is a full definition of the process including:
- The involved stakeholders
- The patient journey including use flow of the intended system
- The use environment
- Motivations, needs, opportunities and especially risks in effectively administering treatment.
During this exercise, the patient must be in the centre of all considerations and this is the success criterion to generate a valid value proposition which translates into a positive and beneficial business case. In effect, an effective use case will drive the business case.
Haselmeier’s disposable D-Flex pen for subcutaneous injection is a good example of how the injection treatment success rate could be increased by following a market and use case-driven, patient-centred approach. The identification and understanding of the involved stakeholders, patient journey, needs and risks led to a unique device platform not currently available on the market.

Figure 1: The D-Flex injection pen is designed for the administration of a small number of selected doses.
HASELMEIER’S D-FLEX SYSTEM
D-Flex (discussed further in this issue, pp 82-86) is a manual injection pen platform for the administration of a small number of selected doses (Figure 1). But what makes the D-Flex a unique manual injection pen?
In some therapies, a finely adjusted variable dose is specified. Typically, a variable dose injection pen will be used for that kind of application. However, those injection devices have myriad injection volumes for the patient or healthcare practitioner to use accurately. Presenting too many choices opens up significant potential for use error in applying the wrong dose. Other therapies require just one fixed dose. In this case a fixed-dose injection pen is used. However, an increasing number of therapies prescribe a limited number of fixed doses. The emergence of biologics and targeted, patient-specific treatment regimens accentuate that need.
So, fixed-dose pens are limiting, while variable-dose pen injectors have such a broad array of dose choices that this may create confusion and errors. Haselmeier saw this as an opportunity to strike a new path and leverage the needs of different kinds of therapies, pharmaceutical customers and patients. The D-Flex manual injector pen platform brings the worlds of fixed-dose and limited variable-dose pens together with a design that makes it very easy to select one fixed or several fixed doses on the same device and includes a mechanism which prevents the selection of unintended doses.
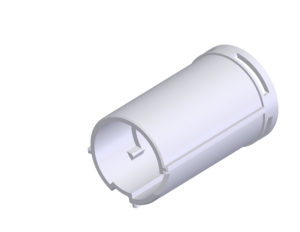
Figure 2: The D-Flex dose selector means there is just one internal component that determines the dose regimen.
The final D-Flex solution reveals just one internal component which determines the dose regimen – the dose selector (Figure 2). Each specific dose regimen can be easily accommodated by only changing the dose selector.
Additionally, the internal D-Flex torsion spring reduces misuse and incorrect dose selection by using the tactile feel of a distinct “click”, thereby significantly reducing the potential error rate. That means that users cannot set a dose between two fixed doses as no clicks secure these in-between doses and the torsion spring turns the setting back to the next lower dose which is built into the device. It represents a significant design contribution to reducing potential use errors.
From a business perspective, D-Flex offers a flexible platform that accompanies customers through their entire product development process, product launch, platform lifecycle phases and even in cases of new applications. The dose setting can easily be adjusted throughout every phase of the clinical study with the one-part change in the pen assembly. Customers can use one variant in Phase II and another variant in Phase III of the clinical trials. The attractive usability features of the D-Flex remain unchanged across that clinical continuum. The D-Flex platform also allows for variation in total volumes in the pen assembly to accommodate a larger number of potential drug options. This level of flexibility reduces risk, saves money and reduces time to market.
A HUMAN FACTORS STUDY OF THE D-FLEX SYSTEM
“The encouraging results of the D-Flex human factors study confirm the importance of the early research and show the value of gathering a deep understanding of the corresponding use case.”
Early on in the innovation project, the design was tested in a human factors study (HFS) to consider potential design changes and concept adaptions to be integrated in corresponding design loops.
One of the potential applications of D-Flex device is for the delivery of GLP-1 receptor agonists such as liraglutide in diabetes. Liraglutide is taken daily starting at 0.6 mg for one week to help gastrointestinal tolerability then moving to a maintenance dose of 1.2 mg, or 1.8 mg depending on the need for further glycaemic control. D-Flex is an attractive device to use for this application as the three doses could be set in the factory using the previously described dose selector so that patients could not select a dose in-between one of these three specified doses.
Aim
Haselmeier elected to run an HF study to assess the usability of the dose-setting feature and to check for usability risks. The priority of the study was to assess the dose-setting, dose-correction and injection force features. Other features addressed in this study were:
- Patient training
- Contents and presentation of the instructions for use (IFU)
- Overall handling of the device (including flow check and needle management)
- Quality and force of dose-setting click
- Legibility of dose scale, dose scale font size
- Design (e.g. size, appearance, anti-rolling feature)
- Cartridge container printing
- Flow check symbols and symbol between set doses
- Patient last dose approach (i.e. what happens if there is insufficient drug to complete their dose).
The aim was to inform Haselmeier of the overall functionality of the pen and identify potential improvements related to specific findings.
Method
The study of D-Flex assessed functional samples and the IFU. The participants were made up of 17 Type 2 diabetes patients. Ten patients used GLP-1 injectable drugs and seven patients were naïve to GLP-1 treatment (four were injection naïve, two were insulin users and one was using an injectable growth hormone therapy). The participant acquisition also considered patients with some level of visual impairment or some level of dexterity impairment or a combination of both. Four diabetes specialist nurses (DSN) were recruited to give their professional opinions on the usability of the device.
The study consisted of 60-minute individual in-depth interviews with the patients, which included several injections with the D-Flex device into an injection pad. A 45-minute individual interview with each nurse was conducted at the beginning and end of the study days, and the nurses observed the patient interviews.
Results
Overall, the D-Flex pen was well received by nurses and patients. It was relatively straightforward for nurses and patients to use D-Flex for the delivery of GLP-1 drugs. Users found the dose setting and correcting easy and agreed that the inability to select intermediate doses was a good safety feature. The nurses also agreed that the dosing mechanism could prevent dose errors.
Furthermore, patients liked the distinct setting of the dose and the proper click sound and haptic feel was perceived as reassurance that the set dose is correct. Users became familiar with the device after a short time and the handling of the pen was considered easy to dial, to set and correct doses.
Some smaller remarks were received with regards to making the design and content of the IFU less complex and providing clearer instructions for the flow check. The encouraging results of the D-Flex HF study confirmed the importance of the early research and show the value of gathering a deep understanding of the corresponding use case by considering all relevant stakeholders to work on the right solution which serves the hidden needs.
CONCLUSION
Innovative applications of human-centred engineering principles to the devices that administer biologics, biosimilars and other novel and existing drugs, will serve to accelerate the already rapid emergence of these molecules onto the market.
With patients now taking an increasingly active role in their care, the need to focus on reducing errors in medication delivery has never been greater. Incremental yet meaningful improvements in delivery systems devoted to reducing use errors is vital. The next wave of user centric drug delivery designs like Haselmeier’s D-Flex will certainly make a meaningful impact in that quality of patient care.
REFERENCES
- “To Err is Human: Building a Safer Health System”. US Institute of Medicine, November 1999.
- “Preventing Medication Errors”. US Institute of Medicine, July 2006.
- “Guidance for Industry; Safety Considerations for Product Design to Minimize Medication Errors”. US FDA, April 2016.
- “Guidance for Industry and FDA Staff; Applying Human Factors and Usability Engineering to Medical Devices”. US FDA, February 2016.
- “Draft Guidance for Industry and FDA Staff; Human Factors Studies and Related Clinical Study Considerations in Combination Product Design and Development”. US FDA, February 2016.
- “Draft Guidance for Industry; Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors”. US FDA, April 2013.

