Citation: Schneider A, “How Users Distinguish Between Self-Injection Device Platforms”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 24-28.
Andreas Schneider highlights how the ever-increasing interest in device platforms raises concerns around the broad availability of lookalike autoinjectors that may result in inappropriate medication usage. He summarises a recent empirical study1 providing insights into which device attributes drive device distinguishability.
“One concern raised by industry experts is that the increasing adoption of platforms may heighten the risk of medication errors.”
Self-injection device platforms have come a long way in disrupting the traditional device development process. In fact, they resolve long-standing industry challenges. Not only do platforms provide attractive cost structures and proven handling concepts across user groups but they also reduce technical risks and speed up time to market. A platform is referred to as a user-tested drug delivery system that, by design, enables the efficient development and manufacturing of drug-specific product variants. It comes as no surprise that most of the recently approved handheld autoinjectors are derived from device platforms (Table 1).
| <2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
| Aranesp® Amgen |
Sumatriptan Sun Pharma |
Otrexup® Antares |
Bydureon® AstraZeneca |
Cosentyx® Novartis |
Benepali® Biogen |
Benlysta® GSK |
Actemra® Roche |
Copaxone® Teva |
| Enbrel® Amgen/ Pfizer |
Rebif® Merck |
Plegridy® Biogen |
Praluent® Sanofi |
Erelzi® Sandoz/ Novartis |
Kevzara® Sanofi |
Aimovig® Amgen/ Novartis |
Cyltezo® Boehringer Ingelheim |
|
| Neulasta® Amgen |
Tanzeum® GSK |
Repatha® Amgen |
Orencia® BMS |
Makena® AMAG |
Amjevita® Amgen |
Epinephrin/ Teva |
||
| Trulicity® Lilly |
Sumatriptan Antares |
Emgality® Lilly |
Fasenra® AstraZeneca |
|||||
| Taltz® Lilly |
Hadima® Merck |
Gvoke HypoPen® Xeris |
||||||
| Zembrace® Promius |
Hulio® Mylan |
Nucala® GSK |
||||||
| Zinbryta® Biogen |
Hyrimoz® Novaris |
Teribone™ Asahi KASEI |
||||||
| Imraldi® Biogen |
Vyleesi® AMAG |
|||||||
| Xyosted® Antares |
Table 1: Non-exhaustive list of approved platform-based disposable single-use autoinjectors (compiled in December 2019).
The significant interest in self-injection device platforms, however, conceals certain reservations. One concern raised by industry experts is that the increasing adoption of platforms may heighten the risk of medication errors. Much is at stake. The emergence of lookalike devices might, experts worry, provoke preventable events causing inappropriate medication usage, potentially putting patients at risk.
“We undertook a non-interventional simulated usage study1 where participants assessed the similarity of autoinjectors.”
On the one hand, patients with multimorbidity increasingly self-manage complex medication plans that could include more than one version of an injection device platform. On the other hand, the complexity of dosing regimens continues to increase and, for instance, may involve the same drug delivery device platform across dose strengths, often distinguished by label information and colouring only.
Unfortunately, we know very little about how users distinguish between self-injection devices. Although research has repeatedly put labelling and packaging of solid oral dosage forms under the microscope to avoid inappropriate medication use, there is limited empirical evidence on what drug delivery device attributes drive users’ ability to distinguish between platform device versions.
We also lack understanding of how various user characteristics – such as professional background, age, dexterity or visual impairments – shape their perceptions and similarity ratings. This matters because platform devices are increasingly used across chronic disease states where device differentiation should be carefully adjusted to specific patient needs and characteristics.
THE STUDY DESIGN SEARCHING FOR ANSWERS
In searching for answers, we undertook a non-interventional simulated usage study1 where participants assessed the similarity of autoinjectors. 74 participants among patients across chronic disease states, nonprofessional caregivers and healthcare professionals rated the similarity of eight autoinjector platform variants.
These device variants differed across four design dimensions that are typically adjusted during customisation work between device manufacturers and pharmaceutical firms: the colour of the label (grey, yellow, orange), the colour of the needle shield (grey, orange, yellow), the overall size (1.0 mL and 2.25 mL prefilled syringe formats) and the device shape (round or square). Eight different autoinjector configurations were included. Each participant thus assessed the similarity of 28 device pairs.
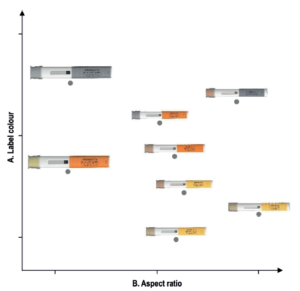
Figure 1: Typical solution space where the distance between autoinjectors reflects participants perceived device similarity. The two dimensions correspond to two design attributes – label colour and aspect ratio – empirically identified to drive device similarity (total sample, n=74).
Multidimensional scaling analysis then transformed these individual ratings in solution spaces to empirically derive the attributes driving how participants distinguish platform device variants. Fuelled by a powerful computational algorithm, this statistical technique allows determination of the underlying dimensions on the basis of individual similarity perceptions – without the need for participants to articulate the rationale for their rating.
Much like drawing a map using the distances between pairs of cities, the algorithm produced solution spaces where the distance between devices corresponds to their perceived similarity: the closer the devices were positioned, the higher their perceived similarity. Figure 1 shows a typical solution space generated by the study. Using a systematic multi-step coding procedure, we then assigned a specific device attribute, or a combination thereof, to each of the emerging dimensions for each resultant solution space.
FIVE DEVICE ATTRIBUTES DRIVE AUTOINJECTOR DISTINGUISHABILITY
First and foremost, the results show that, regardless of their apparent similarity, users are still moderately-to-well able to distinguish between platform device variants. More than half of the similarity ratings (50.3%) were above four on the nine-point Likert scale. Moreover, the simulated use study empirically derived five attributes driving device distinguishability across user groups: the label colour, the size and shape of the device, its aspect ratio and chromaticity. Table 2 illustrates the empirically derived device attributes relevant for device distinguishability.
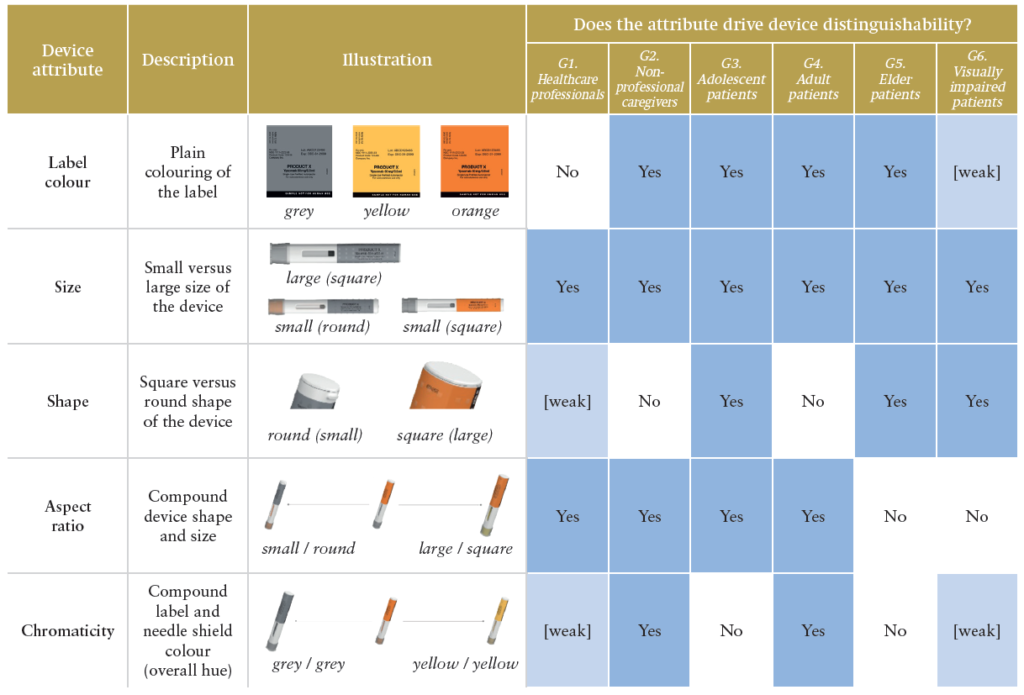
Table 2: Empirically derived device attributes driving platform device distinguishability.
Although label colour, size and shape were anticipated to drive users’ similarity ratings, aspect ratio and chromaticity did not correspond to single design features but highlighted interaction effects between them. First, aspect ratio was the combination of the autoinjector size and shape. Second, chromaticity represented the overall device hue or brightness along the continuum, with the configurations “grey label and needle shield” and “yellow label and needle shield” as its two ends.
“YpsoMate Design
offers fully customised
autoinjectors with
specific individual outer
shapes produced on
the standard platform
manufacturing line.”
These findings hold important implications for device development. The participants did not necessarily distinguish user interface elements but used the overall device appearance, such as its chromaticity, as a basis for similarity ratings. Future device design development thus should integrate different units of analysis, considering potential interaction effects between distinct user interface elements. The results also show that colouring of the needle shield did not emerge as a single device attribute driving device distinguishability. Although needle shield colour is typically modified as part of the routine customisation work, participants did not use this element in isolation to distinguish platform device variants.
Overall, the study suggests geometric features take precedence over tested colour schemes of a specific attribute driving distinguishability. Future device development programmes thus may not only differentiate through colouring a single user interface element, such as the needle shield, but also more holistically adjust colour schemes – including colouring of the label, housing and needle shield – or even pursue individual industrial design options. For instance, YpsoMate Design offers fully customised autoinjectors with specific individual outer shapes produced on the standard platform manufacturing line. From a device development perspective, YpsoMate Design offers the best of both worlds: leveraging the proven platform while enabling full differentiation with the help of unique design shells (Figure 2).

Figure 2: YpsoMate Design leverages the autoinjector platform advantages while offering high industrial design flexibility on the basis of product-specific design shells.
USER CHARACTERISTICS AND USE CONTEXT MATTER
The study provided insights into user group-specific patterns and how device similarity was perceived. Table 2 summarises which design attributes were found to drive similarity ratings per user group. Interestingly, some patterns were linked to user group characteristics (e.g. age, professional education, dexterity and visual impairments) and device usage context.
Elderly patients, for instance, did not use aspect ratio – an attribute linked with the user’s sense of touch and perceived ease of holding the device – as the basis for recognising the device. Their emphasis on visual instead of tactile attributes may be linked to decreasing dexterity with age. Elderly patients may well be aware of, and thus compensate for, decreasing dexterity, thereby prioritising visual over tactile attributes. Similarly, visually impaired patients largely excluded device attributes related to colour (i.e. chromaticity) as the basis for device distinguishability.
The results also detail how educational backgrounds and situational factors influence how users distinguish autoinjector variants. Unlike non-professional caregivers, healthcare professionals primarily used geometric features to assess device similarity. Neither label colour nor chromaticity was relevant for healthcare professionals. These findings re-emphasise prior work where this user group raised concerns over limited time to become familiar with label colouring schemes to identify drug products correctly.
These challenges are particularly pronounced in the context of complex clinical trials where label colouring may be used to convey information about investigational drug type or dosage strength. Here, innovative auxiliary technologies may foster not only effective but also efficient device identification at the point of use.
For example, the reusable cloud-connected sensor module SmartPilot for YpsoMate (Figure 3) automatically identifies the drug product using near-field communication (NFC) tags embedded in the autoinjector label. Using both visual and acoustic feedback, the connected system then notifies users about the correctness of the drug product at hand, thereby reducing the administrative burden at clinical trial sites and confirming allocation of the correct investigational drug to the correct treatment arms.
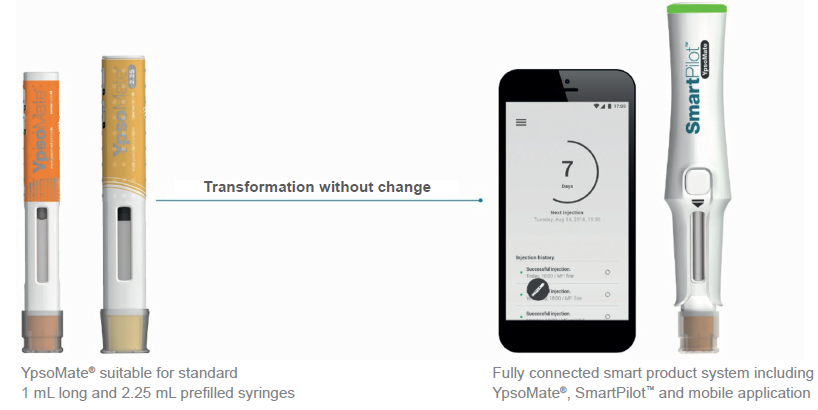
Figure 3: SmartPilot for YpsoMate is a reusable monitoring add-on to transform the marketed disposable two-step autoinjector into a cloud-connected system. It not only tracks injection events and provides real-time guidance to patients but also authenticates the drug product at the point of use.
CONCLUSION
The study summarised here offers much needed insights into how user groups distinguish potentially lookalike platform derived autoinjectors. The results guide future device development toward device attributes that support device distinguishability – namely device size, shape, label colour, aspect ratio and chromaticity. The methodology provides the pharmaceutical industry with a novel toolbox which helps to avoid medication errors through effective device differentiation.
Revealing user group-specific patterns to device distinguishability, the study also suggests it is worth adjusting device differentiation to the intended user population, bearing in mind their characteristics (e.g. age, educational background, dexterity or vision impairments) and the context of device usage. In so doing, it provides the basis for more informed decision making to improve platform device distinguishability and mitigate inappropriate medication use.
The empirical study summarised here was funded by Ypsomed and conducted in collaboration with HFC Human-Factors-Consult (Berlin, Germany).
As a leading developer and manufacturer of mechanical and cloud-connected autoinjectors and pen systems for self-administration, Ypsomed has established the Scientific Research & Communications programme. Its objective is to advance new insights into self-injection devices relevant to industry and academia. The results regularly appear in peer-reviewed scientific forums such as Expert Opinion on Drug Delivery and Medical Devices: Evidence and Research and are presented at leading medical device and drug delivery conferences.
REFERENCES
- Schneider A, Kolrep H, Jordi C, et al, “How to prevent medication errors: a multidimensional scaling study to investigate the distinguishability between self-injection platform device variants”. Exp Opin Drug Del, 2019, Vol 16(8), pp 883–894.

