Citation: Vos W, Pellegrino D, Haley R, “Vectra® MT® LCP Brings the Advantages of Liquid Crystal Polymers to Wearable Injectors”. ONdrugDelivery, Issue 124 (Sep 2021), pp 15–18.
Wim Vos, Dave Pellegrino and Rob Haley discuss the myriad benefits of Vectra® MT® LCP, Celanese’s medical-grade liquid crystal polymer, for wearable injection devices, including improved patient comfort, greater design flexibility and reduced processing costs.
“These characteristics allow LCPs to be used to reliably and accurately produce highly complex designs. Couple this with the ability to produce thin, lightweight, high-stiffness components, and using LCPs for wearable injectors becomes a sensible and attractive option.”
Over the past several years, thinking in the drug delivery industry has seen a shift away from traditional clinic-based practices and towards a patient-centric model. This shift in thinking manifests in a multitude of ways, from an increasing focus on human factors in device design to a push towards introducing digital connectivity functionality, such as smartphone companion apps, to the world of drug delivery devices. A major result of this shift has been the rise of wearable on-body injection devices.
With a goal of increased patient convenience, whilst also tackling the conundrum of delivering high-dose, high-viscosity biologic drug formulations, wearable injectors have proven capable of delivering high volumes of formulation over an extended period of time. This method of injection offers a number of advantages, including significantly reducing the frequency of injections that a patient requires and enabling patients to self-administer at home.
However, designing an injection device to be worn on a patient’s body poses its own unique design challenges. Wearable devices must be evaluated for patient comfort because, unlike any other drug delivery device, a wearable injector is adhered to a patient’s body during use, often for extended periods of time. As such, these devices must be made from material that is strong and lightweight to impose a minimum of burden on the patient, and preferably be as small and discrete as possible to minimise any disruption of their daily lives. A solution presents itself in the form of liquid crystal polymers (LCPs), a polymer material perfect for the device miniaturisation desirable for wearable injectors.
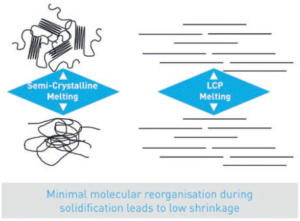
Figure 1: LCP molecules form fibrils that align in the flow direction of the material.
WHAT IS LCP?
LCPs are a family of high-performance polymers formed of rigid, self-aligning molecules. LCP molecules are shaped like a crankshaft and align with each other in concentrated bundles, resulting in fibrils that orient themselves in the direction of flow while in a liquid state and present only a small change in structure when transitioning between liquid and solid (Figure 1). This leads LCPs to act as a self-reinforcing resin or “liquid wood”. LCPs retain this highly crystalline structure until they reach their decomposition temperature.
This property of LCPs results in material properties that make it ideal for use in wearable injectors. Of key significance is that the stiffness of an LCP component increases as the material is made thinner (Figure 2). This means that components can be made as thin as 0.3 mm without sacrificing stiffness; in fact, thinner walls result in stiffer parts.
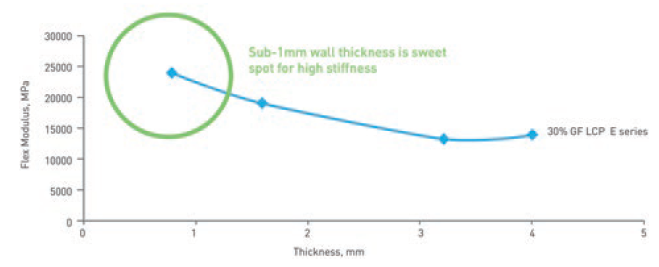
Figure 2: LCP is stiffest when the material is thinner than 1 mm.
“The material properties of LCPs provide significant advantages over other traditional thermoplastics when it comes to processing the material.”
LCPs also boast exceptional flowability when compared with amorphous or semi-crystalline polymers. This ultra-low viscosity has numerous benefits for the production of polymer parts. Additionally, the low latent heat of fusion of LCPs provides three key benefits:
- Fast processing
- High accuracy
- Very low tendency to flash.
These characteristics allow LCPs to be used to reliably and accurately produce highly complex designs. Couple this with the ability to produce thin, lightweight, high-stiffness components, and using LCPs for wearable injectors becomes a sensible and attractive option.
Also of note is that LCPs are remarkably stable. They are environmentally resistant to heat, chemicals, weather and radiation; have low moisture absorption (0.03–0.1%); are inherently flame retardant; have excellent barrier properties to both oxygen and moisture; and operate with a long-term service temperature of -196 – +240°C (340°C in the short term). Furthermore, LCPs have excellent dimensional stability with low shrinkage.
Celanese is able to bring these benefits to the drug delivery device industry with its Vectra® MT® LCP. With decades of experience in the industry, Celanese can provide expert advice on its Vectra MT portfolio to best match the LCP grade to a customer’s requirements, including varying viscosity and tribology, and offer a keen understanding of how using an LCP rather than a more traditional “default” polymer would best benefit a project.
BENEFITS OF LCPS FOR WEARABLE INJECTORS
Strong, Lightweight Material
First and foremost, Vectra MT LCP enables wearable device designers to get the most out of their design while prioritising patient comfort. The tight tolerances and ability to use thinner components without sacrificing stiffness means devices can be made smaller and lighter while maximising the space available inside the device for the necessary injector components and primary drug container. This provides the dual benefit of increased patient comfort and greater design freedom.
Celanese has a broad portfolio of grades of Vectra MT LCP to suit the particular needs of a project, varying the mechanical, dimensional, thermal and tribological properties of the material as necessary. This makes Vectra MT LCP the material of choice for wearable device designers looking to miniaturise their devices, making them more comfortable and discrete for the patient, without sacrificing the quality of the device or compromising the design.
Easy Integration of Electronics
Integrating electronics and connectivity is a widespread trend in the drug delivery industry, and wearable devices are no exception. Many innovative wearable devices integrate connectivity, but even those that don’t frequently incorporate an electronic component to control various aspects of the injection. LCPs are already a widely used material in the consumer electronics industry, as they have humidity-stable dielectric properties, making them the material of choice for micro-connectors and precision optics.
Vectra MT LCP combines Celanese’s MT portfolio service package with a medical grade version of the LCP that has been tried and tested in the consumer electronics sector. Celanese discussed the value of LCPs for the integration of electronics into connected medical devices in greater detail in an article in ONdrugDelivery’s June 2021 issue on Connecting Drug Delivery, and that value can be readily applied to wearable injectors.
Tight Tolerances for Micro-Moulding
As mentioned previously, miniaturisation is a key consideration for wearable injectors, so complex micro-moulded parts can be critical to developing such devices. Material choice makes all the difference for manufacturing small, complex parts, as the material needs to be suitable for fine detail and strong enough to be reliable in use. As such, the tight tolerances and high stiffness at low thickness of Vectra MT LCP make it ideal for use in these complex micro-moulded parts.
High Processability
“The factors that make LCPs so much easier to process naturally result in lower costs and improved sustainability. Rapid cycling of the injection moulding process and higher number of cavities per mould mean that the production rate per tool is significantly higher with LCPs than with traditional thermoplastics.”
The material properties of LCPs provide significant advantages over other traditional thermoplastics when it comes to processing the material. LCPs flow exceptionally well under high shear without degrading their mechanical properties. This high flowability allows LCP to be moulded into very thin, highly complex parts with relative ease; depending on grade, Vectra MT LCP can achieve a flow length of 65 mm at a wall thickness of 0.2 mm.
LCPs also have a low heat of fusion due to their highly ordered molecular structure. As discussed previously, the structure of an LCP changes relatively little between the liquid and solid phases. This means that not only is LCP easy to process, it is fast as well. By using an LCP, the cycle time from melt injection to part ejection can be significantly reduced.
The rapid solidification of LCPs allows for minimal part flashing, which significantly increases the efficiency and reliability of processing. The low flash is also critical when producing small, complex components that are often key to wearable injector designs; low flash means fewer parts rejected for not meeting the precise dimensional requirements.
Typically, LCPs solidify very fast, meaning the injection moulding cycle is commonly 5–15 seconds for small part moulding, depending on the number of cavities in the mould. This leads to higher productivity, as rapid cycling means more parts can be produced in a single mould per unit of time, reducing the number of moulds necessary for the same output. The high flowability of LCPs means that there can be a greater number of cavities per mould, further enhancing productivity.
In contrast with other commonly used thermoplastics, LCPs render high mould temperatures unnecessary, since high shear is used to thin the resin and make it flow better. LCPs can be processed at mould temperatures below 100°C and only require water-based cooling.
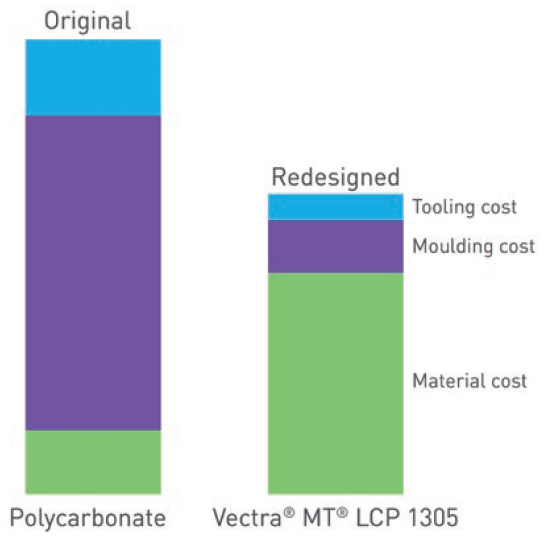
Figure 3: While the material cost of Vectra MT LCP is higher than commonly used polycarbonates, the significant cost savings resulting from its superior processability make it the lower-cost option overall.
Lower Production Costs and Improved Sustainability Profile
The factors that make LCPs so much easier to process naturally result in lower costs and improved sustainability. Rapid cycling of the injection moulding process and higher number of cavities per mould mean that the production rate per tool is significantly higher with LCPs than with traditional thermoplastics. The lower temperatures reduce the energy required for running the process, which, in turn, reduces costs and makes production using hot runners more feasible, which reduces waste, reduces cycle time and provides greater design flexibility.
These advantages mean that, despite the higher cost of the material itself, Vectra MT LCP can be the lower-cost option overall, compared with commonly used polycarbonates (Figure 3) while also providing the myriad benefits to processability and product quality already discussed.
Naturally, many of these cost-savings translate into an improved sustainability profile for parts made using Vectra MT LCP. For example, the lower energy cost per part directly reduces the carbon footprint of devices made using Vectra MT LCP. The high flowability and lower number of moulds required also allows for smaller machines with lower material requirements, which can, coupled with the reduced material waste from the low flash tendency and ready use of hot runners, further reduce the environmental impact of a device using Vectra MT LCP over other thermoplastics.
CONCLUSION
It is critical in drug delivery device design to use the right plastic for the right product. When it comes to the growing field of wearable injectors, an advanced LCP polymer provides a host of benefits over the traditional thermoplastics that designers may default to simply because that is what they have always worked with in the past. As such, it’s important to consult with a materials specialist to take advantage of a more suitable polymer for your product development process. The benefits that using an LCP can provide include:
- Excellent dimensional stability and tight tolerances
- Fine detail frequently unachievable with other materials
- High stiffness at material thickness of less than 1 mm
- High environmental resistances
- Strong barrier properties to both oxygen and moisture
- High processability
- Low flash tendency
- Tried and tested value for integrated electronics
- Significantly reduced processing costs
- Improved sustainability profile.
Celanese is able to provide that expertise and work with device designers to ensure that they’re using the optimal polymer for their device. The company’s MT portfolio service package guarantees material compliance with US FDA and EU requirements, assurance of long-term supply without a change to material formulation and support with regulatory approval. Vectra MT LCP brings together all these advantages, making it the natural fit for any wearable device designer looking to miniaturise their device, improve patient comfort, increase design flexibility and reduce processing costs.
MT® and Vectra are registered trademarks of Celanese Corporation.

