To Issue 129
Citation: Oakley T, “Drug Delivery Device Trends for 2022”. ONdrugDelivery, Issue 129 (Feb 2022), pp 12-14.
Tom Oakley looks at some of the current trends in the drug delivery industry and projects some of the key factors that will influence drug delivery decision making in 2022.
“We can be confident that funding into vaccine development, and the devices to deliver vaccines, will continue to be high for the foreseeable future.”
The drug delivery industry depends on innovation, and keeping abreast of the latest trends can help us predict the future. Springboard is in the privileged position of being at the centre of many of the most exciting drug delivery device developments and has compiled the following list of top trends for 2022.
CONTINUED COVID-19 DIVIDEND
Countries around the world are still struggling to manage waves of covid-19 infections. The highly infectious Omicron variant has undone some of the progress made by the vaccination campaigns conducted in the wealthier economies. Nevertheless, the suffering and disruption brought by covid-19 has led to some profound changes and benefits for the healthcare industry, which Springboard has termed “the covid-19 dividend” (Figure 1).
Figure 1: The ongoing covid-19 crisis has led to some profound beneficial changes in the healthcare industry, which Springboard has termed “the covid-19 dividend”.
Before covid-19, political leaders may have considered vaccine strategy to be primarily concerned with public health. However, the continuing threat of covid-19 is likely to remind them of the vital role healthcare plays in national security and economic welfare too. National governments and supranational organisations, such as the European Union, seem to announce new vaccine procurement deals almost daily.
“Pharmaceutical companies and device manufacturers are looking for ways to reduce the waste going to landfill, and one of the strategies is return-to-manufacturer schemes.”
The US Vaccines National Strategic Plan (published on January 19, 2021) says that “there remains a significant need for new and improved vaccines and vaccination strategies, such as the development of new vaccines against vector-borne diseases, a more broadly protective universal influenza vaccine, and improved strategies for maternal immunisation. Developing new vaccines includes modernising the [US] domestic vaccine enterprise to be highly responsive, flexible, and scalable; improving capacity for agile and rapid responses to emerging influenza threats; and developing more broadly protective vaccines.”1 Therefore, we can be confident that funding into vaccine development, and the devices to deliver vaccines, will continue to be high for the foreseeable future.
Vaccines are not the only area of healthcare in great demand due to covid-19 and the heightened awareness of other potential pandemics that has come with it. Diagnostics (point of care and laboratory), critical care equipment and personal protective equipment are likely to benefit strongly from the covid-19 dividend too.
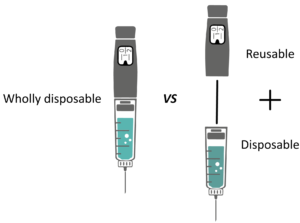
Figure 2: A wholly disposable versus a part disposable, part re-usable pen injector.
RENEWED DEMAND FOR RE-USABLE DEVICES
Many categories of drug delivery device can be developed as either:
- Disposable
- Part re-usable and part disposable (Figure 2).
There seems to be a trend whereby pioneering devices tend to be partly re-usable, after which wholly disposable versions appear and take a share of the market. Examples include:
- Pen injectors, where the earliest model (NovoPen, Novo Nordisk) was re-usable and then disposable models (FlexPen, Novo Nordisk; SoloSTAR, Sanofi; KwikPen, Eli Lilly; FlexTouch, Novo Nordisk; etc) appeared.
- Dry powder inhalers, where the earliest models (Aerohaler, Boehringer Ingelheim; Spinhaler, Fisons; etc) were re-usable2 and then disposable models (Turbuhaler, AstraZeneca; Diskus, GSK; Ellipta, GSK; NEXThaler, Chiesi; etc) appeared.
Many of the devices Springboard is being asked to develop at the moment suggest that the pendulum is swinging back towards partly re-useable devices. Drivers are likely to include:
- Patients and other stakeholders are becoming more sensitive to the environmental impact, especially from plastic waste, of fully disposable devices. Sustainability is discussed in more detail below.
- The current generation of devices tends to be more complicated and costly than the previous generation. For example, on-body injectors are likely to have more components and mechanisms, and therefore have a higher built-in cost than prefilled syringes, safety systems and autoinjectors. The economics of fully disposable devices sets an upper limit on their cost.
- Many new devices have a “connectivity” aspect. The electronics and batteries that are typically involved lend themselves to re-usable modules.
“There have been some innovations over recent years, such as using nitrogen dioxide, but it takes time to validate processes, adapt designs for new sterilisation methods and overcome the risk aversion that is pervasive in the pharmaceutical industry.”
LAUNCH OF MORE RECYCLING SCHEMES
Used drug delivery devices often create biohazard waste that needs to be incinerated and typically some plastic waste as well. Pharmaceutical companies and device manufacturers are looking for ways to reduce the waste going to landfill, and one of the strategies is return-to-manufacturer schemes.
GSK ran a return-to-manufacturer scheme for its inhalers from 2011 to 2020. Sources indicate that take-up was not as high as GSK wanted, although more than two million inhalers were recycled by the scheme. GSK has said that it “believes there needs to be a focus on a wider, joint-working approach across industry, rather than our own standalone approach.”3
Nevertheless, some manufacturers are piloting new schemes, for example:
- Novo Nordisk’s PenCycle pilot was launched on November 1, 2021. If successful, it could be expanded to cover the whole of the UK and could recycle many of the 2.5 million FlexPen and FlexTouch devices sold in the UK each year.4
- Chiesi’s inhaler recycling scheme started in February 2021 and covers any inhaler from any manufacturer. It could reduce the waste caused by the 73 million inhalers prescribed each year in the UK.5
We can expect to see more recycling schemes announced in 2022 if these pilots are successful.
NOVEL STERILISATION TECHNOLOGIES
Ethylene oxide and gamma irradiation methods account for the majority of medical device sterilisation. However, ethylene oxide has a relatively long cycle time (including aeration time to reduce sterilant concentration) and the sterilant is explosive and carcinogenic. On the other hand, gamma radiation can damage materials, turning transparent materials yellow or brown, and crosslinking certain useful engineering polymers such as polytetrafluoroethylene (PTFE) and polypropylene. Gamma radiation can also damage drugs and electronics, and a global shortage of cobalt-60 is limiting capacity.6
There have been some innovations over recent years, such as using nitrogen dioxide (Noxilizer, MD, US), but it takes time to validate processes, adapt designs for new sterilisation methods and overcome the risk aversion that is pervasive in the pharmaceutical industry. However, demand for alternative sterilisation methods will persist through 2022 and beyond.
SUMMARY
More than at any other time, in 2022, the world recognises the need for high quality, accessible and sustainable healthcare. We are likely to see remarkably high levels of investment in vaccines, diagnostics, critical care, personal protective equipment and reducing environmental impact.
If you have questions or would like to discuss any points, please do not hesitate to contact the author.
REFERENCES
- “Vaccines National Strategic Plan”. US Department of Health & Human Services, reviewed Feb 2021.
- de Boer AH et al, “Dry Powder Inhalation: Past, Present and Future”. Expert Opin Drug Deliv, 2017, Vol 14(4), pp 499–512.
- Clews G, “Inhaler Recycling Scheme That Cut Carbon Emissions Equivalent to More Than 8,500 Cars Is Scrapped”. Pharm J, 2020, Vol 305(7939), p 305.
- Wickware C, “First Ever Injection Pen Recycling Pilot Launched in UK Pharmacies”. Pharm J, 2021, Vol 307(7955), DOI:10.1211/PJ.2021.1.112912.
- Robinson J, “Chiesi Launches Postal Asthma Inhaler Recycling Scheme”. Pharm J Online, February 17, 2021, DOI:10.1211/PJ.2021.20208795.
- Craven E, “Gamma Sterilization of Medical Devices”. US FDA Medical Device Advisory Meeting, Nov 2019.

