To Issue 131
Citation: Chandra A, Audibert R “Nasal Delivery System Expands Public and Patient Access to Naloxone to Mitigate the Opioid Overdose Epidemic”. ONdrugDelivery, Issue 131 (Apr 2022), pp 38–40.
Todd D Pizitz and Donald R Mealing discuss the ongoing US opioid epidemic, the CounterAct cap containing a single dose of naloxone to be attached to prescribed opioids, and a recent pilot market study done to gauge public enthusiasm for this life-saving technology.
“There is one overdose intervention strategy that has proven more effective in reversing the effects of opioid-induced respiratory failure than any other – the administration of naloxone during the critical minutes of an overdose emergency.”
The US opioid epidemic continues to persist without much relief in sight. So far, efforts to curb or reduce the death rate from opioid overdoses in the US have not impacted the daily death rate of 255 people.1 Recent research has concluded that almost all US states have poorly developed paradigms for distributing naloxone and estimates that as many as 80% of opioid overdoses occurred in the presence of someone who could have counteracted the overdose with the administration of naloxone.2 This growing death toll demonstrates an urgent unmet need for timely administration of naloxone to reverse opioid overdose.
Statistics show that, in 2020, 142 million opioid prescriptions were filled in the US.3 Tragically, 10 million people in the US misused prescription opioids in 2020–21, resulting in 70,000 deaths from overdose and 500,000 non-lethal overdoses.4 As such, opioid overdose is the leading cause of death for 25-to-64-year-olds in the US,5 with a financial impact of more than US$1 trillion (£760 billion) in 2017.6 So the question remains, what will it take to reverse this disturbing trend?
There is one overdose intervention strategy that has proven more effective in reversing the effects of opioid-induced respiratory failure than any other – the administration of naloxone during the critical minutes of an overdose emergency. In this regard, naloxone administered via a single dose nasal spray has saved countless lives in the hands of emergency first responders. The key question then becomes, “Would greater public access to naloxone prevent more opioid overdose fatalities?”
“The idea behind the CounterAct Cap was to have opioid medications and naloxone paired together, serving as a reminder of the necessity of prescriber compliance and that mismanagement and abuse of opioid medication can lead to death.”
Efforts to expand access to naloxone in the US have resulted in co-prescription laws instructing prescribers who write opioid medications to also consider prescribing naloxone formulations and mandating the co-prescription if any of the following three co-prescription criteria are present:
- A patient receives a prescription for an opioid medication that contains a 90 mL morphine equivalent
- The prescriber suspects a potential for abuse of the opioid medication or the patient has a history of substance abuse
- The opioid medication is also prescribed with a benzodiazepine medication.
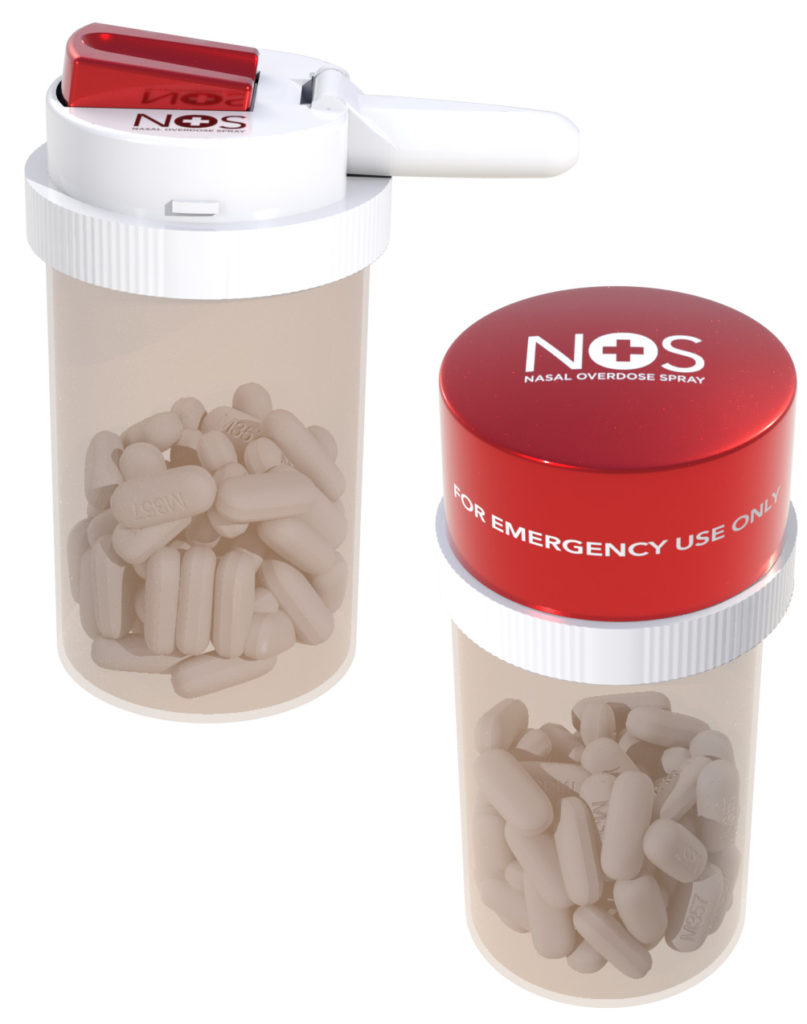
Figure 1: The CounterAct Cap contains a single dose of life-saving naloxone.
In September 2018, California passed a co-prescription law mandating prescribers to co-prescribe naloxone with opioid medications. Other states are following the same pathway to co-prescription of naloxone with opioids. Although co-prescribing naloxone with the opioid significantly lowers the rate of fatal overdose, life-saving naloxone was provided to only 2% of patients at high risk of overdose.7 Nearly 40% of overdose victims were using medically prescribed opioids, most often obtained from a friend, relative or medical provider.8 The most promising approach to reduce opioid overdose is to increase naloxone availability, as promoted by the US Department of Health and Human Services (DHHS) and the US FDA.
Emergency medical services and most law enforcement agencies that serve as first responders have a sufficient supply of and access to life-saving naloxone, however the public does not. To expand naloxone availability to the home- and patient-use market, aligned with the DHHS and FDA’s recommendation to increase the access to naloxone, CounterAct designed the CounterAct Cap. The CounterAct Cap places a single dose of life-saving naloxone on top of the container holding an opioid patient’s prescription pills (Figure 1).
In a suspected opioid overdose emergency, a patient’s family members, friends or associates can instantly administer the naloxone spray after calling 911, thereby saving precious time. The safety cap twists off to reveal a folded nozzle that is easily extended. Once the nozzle is fully extended, it can be placed into the overdose victim’s nose, and the spring-loaded trigger can be pressed to instantly release a 4 mg dose of naloxone to the victim (Figure 2). The idea behind the CounterAct Cap was to have opioid medications and naloxone paired together, serving as a reminder of the necessity of prescriber compliance and that mismanagement and abuse of opioid medication can lead to death.
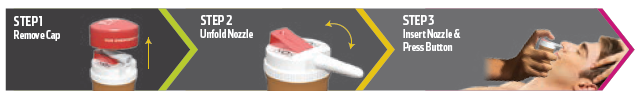
Figure 2: Use instructions for the CounterAct Cap.
In 2021, CounterAct completed a pilot study of residents of several counties in Southern California to determine the market interest in the CounterAct Cap. All participants were shown pictures and videos of the CounterAct Cap to inform them of the device’s utility and purpose. After a brief introduction to the device and personal review of collateral information, participants answered a series of questions.
A total of 43 participants were contacted, of which 29 met at least one of the inclusion criteria and were therefore included in the study. The demographics of the participants are listed in Table 1. The inclusion criteria consisted of experiencing at least one of the following:
- The participant had any experience with a naloxone-based product
- The participant had been involved in an opioid overdose either as a victim, observer or first responder
- The participant had a friend or family member who had overdosed on opioids. The participants meeting the inclusion criteria were asked the following questions:
- Do you think the CounterAct Cap would save lives in an opioid overdose emergency?
– Yes: 29 (100%); No: 0; Uncertain: 0 - Would you want to have access to the CounterAct Cap if you have friends or family who use opioid medication?
– Yes: 27 (93%); No: 0; Uncertain: 2 (7%) - Would you request your pharmacist or doctor to prescribe the CounterAct Cap for friends or family members that are prescribed opioid medication?
– Yes: 27 (93%); No: 0; Uncertain: 2 (7%)
| Age | Gender | Ethnicity | Employment Status | Marital Status |
| 49.8 Years (Mean) |
11 Males (38%) 18 Females (62%) |
22 Caucasian (75%) | 19 Employed (65%) |
19 Married (65%) |
| 2 Latino (7%) | ||||
| 2 African-American (7%) | ||||
| 2 Asian (7%) | ||||
| 1 Native American (4%) |
Table 1: Demographics of market survey participants.
The 29 participants were asked for general impressions of the Counteract Cap. The following is a sampling of statements from the participants:
- “I wish I had this when my son overdosed, rather than waiting for 911.”
- “This could really save lives.”
- “The cap on top of the pills makes it easy to get to in an emergency.”
- “The combination of the two drugs together is great.”
- “Is this available now from my doctor?”
- “Should be a must for every household.”
- “Brilliant.”
- “Great technology.”
- “Where can we get one?”
- “This is awesome.”
The participants in this pilot market interest survey demonstrated an overwhelmingly positive reaction to the CounterAct Cap. Since patient acceptance of the device is paramount for its use, the enthusiastic response from potential victims and family members provides critical support for CounterAct’s goal of providing increased access to naloxone via the CounterAct Cap.
CounterAct has also surveyed those afflicted by the opioid overdose epidemic for their reactions to the Counteract Cap. Cammie Rice Wolf, Founder and Board Chair at Christopher Wolf Crusade (GA, US), who lost her son to an opioid overdose, was surprised that the CounterAct Cap was not in production and being developed due to its life-saving potential. She commented, “I believe the cap is also an answer for rural communities that are sometimes 50 to 100 miles from an emergency medical technician or hospital. In addition, when overdoses take place many times no one wants to call for help because of the ramifications, so they don’t call for help.”
CounterAct was issued a US patent for this life-saving device in March 2021. The company continues to collaborate with major drug and device manufacturers and investors to bring the CounterAct Cap to market.
REFERENCES
- “A Time of Crisis for the Opioid Epidemic in the USA”. Lancet, Jul 2021, Vol 398(102977), p 277.
- Irvine MA et al, “Estimating Naloxone Need in the USA Across Fentanyl, Heroin, and Prescription Opioid Epidemics: A Modelling Study”. Lancet Public Health, Mar 2022, Vol 7(3), pp E210–E218.
- “US Opioid Dispensing Rate Maps”. US Centres for Disease Control and Prevention website, accessed Mar 2022.
- Rzaza Lynn R, Galinkin J, “Naloxone Dosage for Opioid Reversal: Current Evidence and Clinical Implications”. Ther Ad Drug Saf, Jan 2018, Vol 9(1), pp 63–88.
- Skolnick P, “Treatment of Overdose in the Synthetic Opioid Era”. Pharmacol Ther, Oct 2021, Article 108019.
- Florence C, Luo F, Rice K, “The Economic Burden of Opioid Use Disorder and Fatal Opioid Overdose in the United States, 2017”. Drug Alcohol Depend, Han 2021, Vol 218, Article 108350.
- Lin LA et al, “Association of Opioid Overdose Risk Factors and Naloxone Prescribing in US Adults”. J Gen Intern Med, Feb 2020, Vol 35(2), pp 420–427.
- “Prescription Opioid Overdose Death Maps”. US Centres for Disease Control and Prevention website, accessed Mar 2022.

