To Issue 132
Citation: Thomsen C, Werner Hansen P, “Sustainability in Medtech Development: an Iterative Approach Based on Ecodesign and Life Cycle Screenings” ONdrugDelivery, Issue 132 (Apr-May 2022), pp 12–16.
Life Cycle Assessments are a valuable but time-consuming and, ultimately, retrospective method for assessing MedTech product sustainability. Christoffer Thomsen and Peter Werner Hansen dive into an iterative and data-driven approach to more sustainable MedTech development based on Ecodesign and Life Cycle Screenings.
WHY SUSTAINABILITY IN MEDTECH?
Sustainability demands have become ubiquitous in today’s MedTech industry. Regulatory requirements for sustainability are increasing. Moreover, sustainability criteria are becoming commonplace in tender processes within the healthcare sector. Also, patients want to understand the environmental impact of the MedTech products used to improve their everyday lives.
These are just a few examples in the range of sustainability demands currently facing the MedTech industry, as experienced by a number of Technolution’s clients (Figure 1).
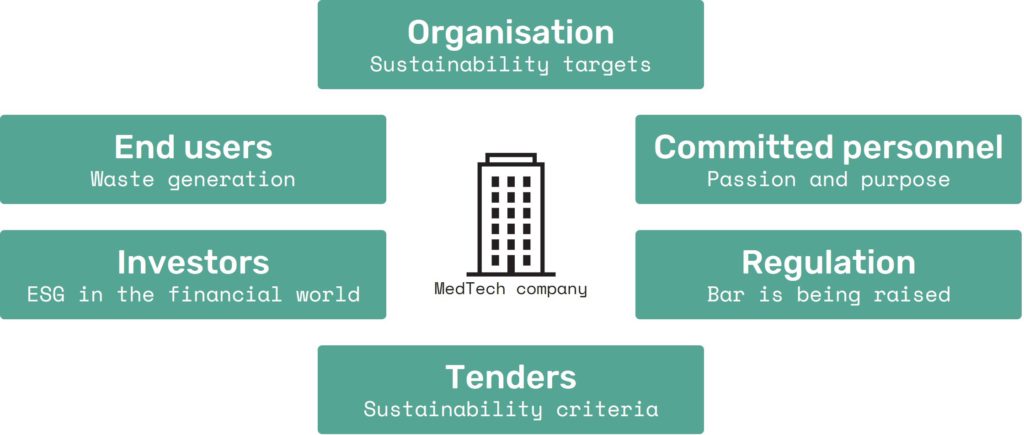
Figure 1: The MedTech industry is faced with a broad range of sustainability demands.
However, making products more sustainable is no simple task for MedTech companies. Development cycles lasting several years and strict regulatory requirements on products and safety, for example, make it challenging to incorporate sustainability in an already complex development process. Furthermore, gaining data about the sustainability performance of a medical device through Life Cycle Assessments (LCAs) is a costly and resource-consuming affair.
Technolution has developed an approach to more sustainable medical device development based on ecodesign principles and life cycle screenings. The approach is firmly based on industry standards and can be applied from the beginning of the development process in a more timely and agile way than LCAs. Rather than being retrospective in character, this approach is useful during all stages of product development. The goal is to bring a proactive approach to more sustainable product development by gaining fast access to dependable sustainability data throughout the development process.
GOING GREEN THROUGH ECODESIGN AND LIFE CYCLE SCREENINGS
“Bridging the gap between complete sustainability knowledge about a MedTech product throughout its life cycle and, on the other hand, being able to use sustainability knowledge actively during development is key to success when making greener medical devices.”
When developing medical devices with a smaller environmental footprint over the entire life cycle of a product, Technolution uses a combination of qualitative and quantitative methods.
Technolution’s ecodesign principles are based on industry best practices as a fundamental and systematic part of the company’s development to analyse and choose the most appropriate ways of achieving more environmentally sustainable medical devices. This is the qualitative approach.
Technolution also uses life cycle screenings based on the LCA methodology in ISO 14040/44 to measure environmental impacts, thereby enabling data-driven decision making throughout the development process. This quantitative approach is key to ensure that sustainability improvements are made to the product with data to support the decisions.
Sustainability engineering should become a “swimlane” of its own during the development process, not just an add-on to existing areas, such as mechanical engineering. This is where ecodesign and life cycle screenings become a key tool for improving and assessing product sustainability during development in a manner that facilitates fast decision making while being based on data all the way. The goal is to enable MedTech product developers to bring more sustainability into the development process easily, from the very beginning, and keep a laser-sharp focus on making green improvements throughout.
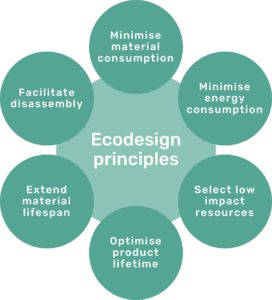
Figure 2: The six Ecodesign principles of Technolution.
ECODESIGN – THE QUALITATIVE APPROACH
Applying ecodesign principles is a systematic approach that considers environmental aspects in design and development with the aim of reducing adverse environmental impacts throughout the life cycle of a product. The approach strives to achieve products with the lowest possible environmental impact throughout the product life cycle without compromising performance, patient safety, functionality, aesthetics, quality or cost.
Technolution uses six ecodesign principles (Figure 2) as a foundation for improving various aspects of sustainability during the development process. The principles align with the sustainability guidelines of the company’s customers, which makes the principles firmly grounded in industry best practices. The principles are also based on established theory from the ecodesign research community.
It is important to note that the six ecodesign principles are not necessarily weighted equally and should not be seen as a checklist where every principle must be addressed. Their priority highly depends on project context – some principles may not be prioritised at all, while others may influence or be in conflict with each other.
However, all ecodesign principles use the same taxonomy based on guidelines. For instance, the ecodesign principle minimise material consumption uses the four guidelines in Figure 3 to specify the areas of focus when attempting to make sustainability improvements in the medical device.

Figure 3: The four guidelines for the Ecodesign principle minimise material consumption.
As can be seen from the guidelines, the goal of the minimise material consumption ecodesign principle is to reduce material consumption as much as possible in relevant stages of the product life cycle. This is not only beneficial from an environmental viewpoint but can also lead to cost savings and support a lean production mindset through, for example, light-weighting or miniaturisation. While reducing material consumption is not new to the industry in general, quantifying the environmental benefits often has not been a priority so far.
Another important aspect of the ecodesign principles is the alignment of circular economy concepts. The facilitate disassembly ecodesign principle is a great example, as this principle ensures that a medical device is designed with the purpose of separating the parts or materials at end-of-life. By using reversible joining systems or only permanently joining parts made from the same material, for example, the materials used for manufacturing can be “resynthesised” and used again as part of recovery at the end-of-life stage (Figure 4).
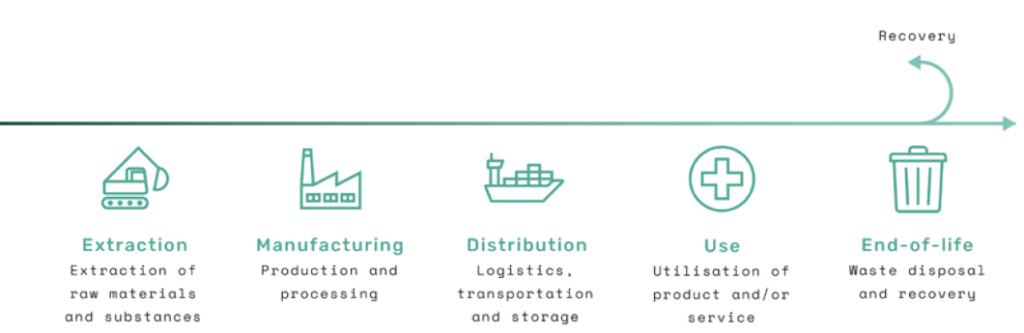
Figure 4: The five generic life cycle stages of a product from extraction of materials to end-of-life.
Picking the right subset of ecodesign principles to focus on during medical device development cannot be done without the proper data acting as support and validation for the sustainability choices being made – this calls for a quantitative approach.
LIFE CYCLE SCREENINGS – THE QUANTITATIVE APPROACH
Making continuous sustainability improvements to a medical device during product development requires specific knowledge about its environmental impact. It is well known in the industry that LCAs are of a retrospective nature and therefore not well-suited for use during product development.
Bridging the gap between complete sustainability knowledge about a MedTech product throughout its life cycle and, on the other hand, being able to use sustainability knowledge actively during development is key to success when making greener medical devices.
Technolution’s life cycle screenings are based on the LCA methodology in ISO 14040/44 and aim to provide the same information as an LCA but in less detail, based on more generic data and assumptions. This makes it applicable during a product development process, providing invaluable feedback to the ecodesign process in an iterative way, as shown in Figure 5.
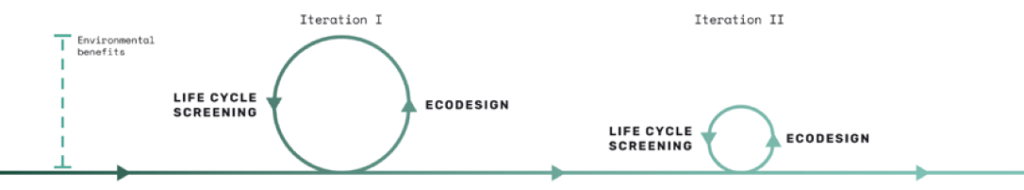
Figure 5: The iterative relationship between Ecodesign principles and life cycle screenings. The earlier a focus on sustainability is implemented in development, the more environmental benefits can be obtained.
The framework for making life cycle screenings contains the same four key steps as an LCA (Figure 6). Initially, Technolution sets a goal and a scope for the screening, such as a hot-spot analysis of the biggest environmental impacts of a product or concept. The next step is to create an inventory for the product, such as the parts in an autoinjector, including, among other things, information about manufacturing processes, packaging and data related to transportation. At this point, the level of detail is not as fine-grained as an LCA but still high enough to produce useful sustainability data. Then an impact assessment can be made by quantifying impacts, followed by a data interpretation step. This is the last step in which assumptions can be revisited and challenged if necessary.
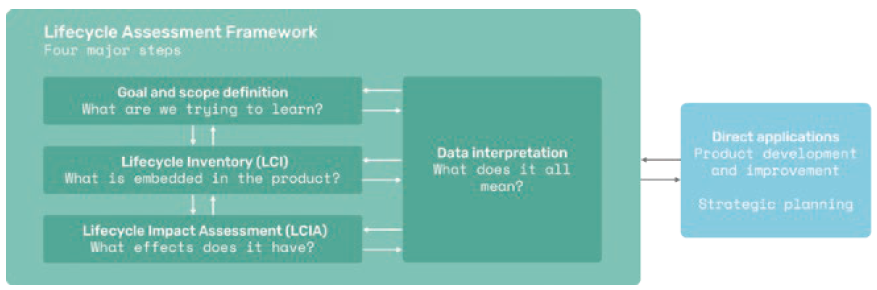
Figure 6: The framework for conducting life cycle screenings is derived from the LCA framework as outlined in ISO 14040.
Results are used with Ecodesign principles to improve sustainability performance. (Source: ISO 14040)
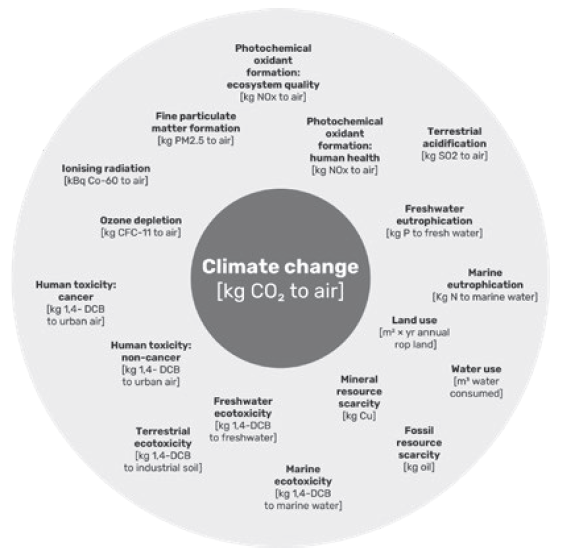
Figure 7: A narrow focus on greenhouse gas emissions and climate change leaves many important environmental issues out of sight, as shown with the ReCiPe 2016 midpoint categories.
If the data interpretation shows that materials are responsible for the biggest environmental impact, for example, it aligns with the ecodesign principles related to improving sustainability based on materials. This creates a foundation for making improvements to the medical device design, assessing the sustainability impacts with another life cycle screening and so forth.
One major pitfall that can occur while making sustainability improvements to a product is to focus too narrowly on climate change. While greenhouse gas emissions are certainly one of the most significant concerns regarding the global ecosystem, many other environmental parameters should also be considered during medical device development. This includes, but is not limited to, freshwater eutrophication, human toxicity, mineral resource scarcity, water use and ozone depletion, as shown in Figure 7. The life cycle screening approach takes these parameters into consideration when doing the calculations for the life cycle impact assessment.
CONCLUSION
This article has discussed a two-pronged approach for making sustainability assessments and improvements during medical device development. The approach combines ecodesign principles with life cycle screenings and brings important aspects from LCAs into the core of the MedTech development process in a manner that is much faster and more agile than LCAs.
The iterative nature of this data-driven sustainability engineering process makes it possible to improve medical device sustainability at an early stage and throughout development. This is key to avoiding cost-intensive product changes at a late stage in the development process and to take the guesswork out of sustainability improvements.
BIBLIOGRAPHY
- McAloone T, Pigosso D, “Ökodesign” in “Pahl/BeitzKonstruktionslehre” (Bender B, Gericke K, eds). Springer Vieweg, Berlin, Heidelberg, 2021.
- Vezzoli C, Manzini E, “Design for Environmental Sustainability”. Springer, London. 2008, pp 1–303.
- Brezet H, van Hemel C, “Ecodesign: A Promising Approach to Sustainable Production and Consumption”. France: UNEP, (1997.)

