To Issue 137
Citation: Estep J, Desai M, “Flexible Care – Enable Wearable Delivery”. ONdrugDelivery, Issue 137 (Sep 2022), pp 18–21.
Jennifer Estep and Mehul Desai discuss the benefits for patients and healthcare providers of innovative subcutaneous delivery.
Anne heard the news from her physician at a young age – she had a chronic disease, one for which there is no cure. She had few options – she could either ignore her diagnosis and live confined to the full progression of her disease or she could aim for remission and learn to live with needles, nurses, an infusion chair and a rigid infusion schedule. Anne did not feel as if she had a choice.
After a few years of intravenous (IV) therapy, Anne found out she was in remission, and was elated. To stay in remission, she continued her therapy, but a change in policy by her insurance company meant she could no longer receive her IV infusions in the clinic. She had to move to home health for her IV infusions. Even though getting IV therapy at home is good news for many patients, as they no longer have to commute, Anne felt as though it was one more thing that she could not control.
The home infusions did not go as planned. The first scheduled home infusion day came, but the medicine and supplies didn’t arrive until the next day. The rescheduled first home infusion day also did not work out, as a different home health nurse got lost and never arrived at her home. The third try was a success, but Anne had new concerns. What happens when the nurse is different every time? And what about the risk of covid-19 exposure by allowing a nurse who had visited other patients that same day and week? Was she comfortable having a stranger in her home every time she needed her infusion?
She realised that, as an immune-compromised patient, she did not want to be exposed to health risks like covid-19 in her home or share her couch and TV shows with a stranger while she received her IV treatment. It was such a concern that Anne informed the insurance company she wanted to go back to the IV clinic because she had more control. The insurance company refused the request, and Anne compromised by agreeing to receive her infusions in the home health company office building. The room where she received her IV therapy was a remote, unfriendly cinder block room. It was not what she wanted, but she felt she did not have a choice. Was there an alternative?
“Innovations in healthcare are imperative for improving access to medicines and the delivery of therapeutics for patients, caregivers and providers.”
GAP IN CURRENT CARE
For patients and healthcare providers (HCPs), the subcutaneous (SC) option is a strong one. However, many drugs are not available for SC delivery, for a variety of reasons.
Parenteral Administration
Many drugs must be administered parenterally, specifically biologics, and a common parenteral route is via IV administration. IV administration requires an HCP to initiate and monitor the infusion, usually takes several hours and involves an exposed needle.
SC administration, another parenteral route of administration, requires a fraction of the time of IV administration. However, SC administration has traditionally faced limitations, such as drug volume limitations, safety concerns due to exposed needles, hands-on administration and lack of mobility during administration.
Drug Volume Limits
SC administration has historically only involved small volumes (<5 mL) of drug delivered by a device through a needle – such as prefilled syringes and autoinjectors. To reach larger delivery volumes (>5 mL), dosing regimens would have to include multiple injections by a syringe or autoinjector per dosing time.
For pharmaceutical formulation teams, volume restrictions are an impediment. By allowing formulations to be delivered in larger volumes by using modern large-volume SC delivery technology, the development time may be reduced (Figure 1).1
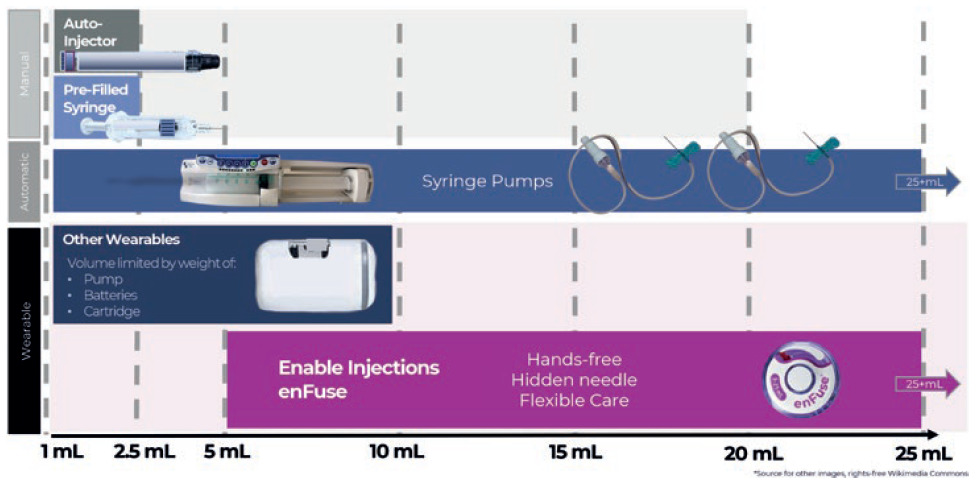
Figure 1: Overview of subcutaneous drug delivery system technology by delivery volume capacity, by type of administration for manual systems and wearable systems.
Exposed Needle Safety Concerns
Dislike of exposed needles is an issue that affects much of the population. In research conducted in a validation study with kidney failure patients receiving treatment, 26% reported feeling afraid, 41% reported feeling nervous and 33% reported feeling worried the “moment the nurse comes to insert the needle”.2
Studies report that approximately 25% of the adult population has a fear of needles, and this percentage is higher among children and adolescents.3 A 2021 review of management of needle fear in adults with chronic disease discusses a study in which 35% of patients reported delaying treatment because of needle phobia due to fear of pain.2
Hands-Free Needs
For small- and large-volume delivery via syringe, the medicine must be delivered to the patient using a hands-on approach. For current large-volume SC delivery via syringe, an HCP sits inches from a patient’s body, actively pushing the barrel of a large-volume syringe. This syringe delivers therapeutics through an exposed needle stuck into the subcutaneous tissue of the patient’s stomach, and typically requires 5-10 minutes of pushing. The focused effort required for this type of injection limits the HCP’s time, and the physical rigour required to administer this type of large-volume injection limits the number of times an HCP can repeat the process in a single day.4,5,6
Large-Volume Delivery Alternatives
One alternative to the large-volume delivery with a syringe is a syringe pump. A large-volume syringe pump removes the need for an HCP to manually inject the medication into the SC tissue. However, the pump does not eliminate exposed needles, tubing sets or the need for the patient to sit immobile throughout the infusion.7
Improvements in Large-Volume Delivery
On-body delivery device volumes are usually limited by device weight, size and container. Current on-body delivery devices are limited to volumes of 10 mL and below. These devices deliver volumes that may also be administered by several self-administered prefilled syringes, autoinjectors or large-volume syringes administered by an HCP.
IMPROVEMENTS THROUGH INNOVATION
Innovations in healthcare are imperative for improving access to medicines and the delivery of therapeutics for patients, caregivers and providers. For patients seeking flexible, convenient care that saves time and fits into their schedules, SC delivery has that potential with large volume, on-body delivery technology that is truly innovative in size, shape and wearability. As one key opinion leader said at the American Society of Clinical Oncology (ASCO) congress in Chicago, IL, US, June 2022, “SC administration by on-body delivery systems is well tolerated, requires short duration of injection and provides a convenient hands-free option.”8
Innovation benefiting patients and providers has the potential to increase flexibility and:
- Reduce needle exposure
- Allow ease of treatment and hands-free administration
- Allow potential flexibility in the location of the administration – in the clinic or at home, based on the patient and provider needs and preferences.
ENFUSE INNOVATION
Enable Injections is an investigational-stage company developing and manufacturing the enFuse wearable delivery system, designed with the patient in mind. The enFuse (Figure 2) is a novel, wearable drug delivery system capable of hands-free delivery of large volumes of large- and small-molecule therapeutics (>5 mL). Its design simplicity includes using a standard container-closure system and a hidden needle, and its mechanical simplicity eliminates the need for a motor, electronics, batteries or sensors. Its flexibility allows administration in a clinic or hospital, at a pharmacy or at home via self-administration by the patient or by an HCP. Its patient-friendly appearance is smaller and lighter because of its unique technology.

Figure 2: The enFuse is a novel, wearable drug delivery system that can deliver large volumes of large- and small-molecule therapeutics (5–25 mL).
For the enFuse user, a simple single click of a vial into the enFuse transfer base initiates the transfer of medication into the enFuse. Once the transfer is completed, the user removes the enFuse from the transfer base, places the enFuse onto the abdomen and presses the button to initiate injection. The needle is never exposed to the user and allows a hands-free injection (Figure 3).

Figure 3: Throughout the enFuse injection, the needle is hidden from the user and allows for a hands-free injection.
enFuse EnywhereCare™ flexibility allows in-clinic or at-home self-administration, based on patient and provider needs. enFuse EnyVolume, with flexibility in volumes from 5-25 mL in a single enFuse device, allows for greater clinical efficiencies for providers. Pharma developers may benefit from EnyVolume with greater flexibility and potentially reduced development time.
Constant Pressure Delivery Advantages
The enFuse constant pressure mechanics promote greater flexibility surrounding the speed of the injection and responsiveness to the pressure in the SC tissue. This responsive constant pressure delivery has several advantages, including the potential to increase patient comfort and potentially reduce pain, leakage and backpressure, as well as potentially eliminating the need for the inclusion of a chemical additive to allow SC absorption, such as hyaluronidase.
CONCLUSION
For patients like Anne, the enFuse may offer more than flexibility and simplicity. SC administration of large-volume therapeutics with enFuse technology has the potential to allow patients like Anne to achieve their goals of staying healthy and in remission by continuing their treatments, but with the discretion and flexibility that would allow them to regain control over their calendar and healthcare. For Anne, this could mean thinking of herself less as a patient and more as a human being able to live, thrive and achieve her dreams.
REFERENCES
- Badkar A et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- Duncanson E et al, “The prevalence and evidence-based management of needle fear in adults with chronic disease: A scoping review”. PLoS ONE, 2021, Vol 16(6), e0253048.
- McLenon J, Rogers M, “The fear of needles: a systematic review and meta-analysis”. J Adv Nurs, 2019, Vol 75(1), pp 30-42.
- US Package Insert, Darzalex Faspro (daratumumab + hyaluronidase-fihj), US FDA Web Page, 2020. (https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761145s000lbl.pdf, Accessed August 2022)
- US Package Insert, Rituxan Hycela (rituximab/hyaluronidase human) US FDA Web Page, 2017. (https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761064s000lbl.pdf, Accessed August 2022)
- Respondent data on file, Enable Injections, 2021.
- Instructions for Use, RMS Freedom Edge Syringe Infusion System, Manualzz Web Page, 2017. (https://manualzz.com/doc/63884633/rms-freedom-edge-instructions-for-use-manual, Accessed August 2022)
- Wentworth S, “ASCO 22 interview: Sanofi innovates in multiple myeloma”. The Pharma Letter, Jun 2022.

