To Issue 142
Citation: Desai M, “Reduce Pharma Development Time Through Large-Volume Subcutaneous Delivery with the enFuse®”. ONdrugDelivery, Issue 142 (Feb 2023), pp 38–41.
Mehul Desai discusses how Enable Injections‘ enFuse® delivery system provides a flexible alternative for subcutaneous administration of high-volume therapeutics.
“With current innovations, it is unnecessary for manufacturers to continue attempting to overcome physiochemical challenges to achieve a small volume (<3 mL) for SC delivery.”
Subcutaneous (SC) drug development presents several challenges, and these challenges are amplified when trying to achieve a low-volume formulation. Intravenous (IV) administration has a bioavailability of 100%, but the bioavailability for SC monoclonal antibody drugs is 60%–80%.1 This means the dose is typically higher for SC delivery than for IV delivery to achieve therapeutic efficacy.
Unfortunately, there is a common and inaccurate belief that SC drug delivery should not exceed a volume of 3 mL per dose.2 Several well-known drugs are available subcutaneously at volumes >3 mL, including, but not limited to, trastuzumab,3 rituximab,4 daratumumab5 and pertuzumab/trastuzumab.6 Despite these therapies being co-formulated with a permeation enhancer (hyaluronidase), they are still administered at quite large volumes, ranging from 5–15 mL and delivered over several minutes.3–6 With current innovations, it is unnecessary for manufacturers to continue attempting to overcome physiochemical challenges to achieve a small volume (<3 mL) for SC delivery. Formulation challenges can be expensive and time consuming, delaying convenient life-saving treatments for patients.
The process for storing a drug for SC delivery is multifaceted and involves several steps. The container that the drug is stored in can vary and requires substantial testing, but most often, biologic drugs are manufactured and stored in glass vials. From beginning to end, this testing is expensive and can take several months; therefore, the chosen SC delivery method can impact time to market, development costs and even commercial uptake. The value of reaching the market faster with a flexible drug delivery format is an obvious benefit and even more pronounced when considering the evolving competitive landscape and limited patent life of a molecule.
While the drug is the foundation of treatment, the delivery method of the drug is critical for patient preference, as some methods of SC delivery allow for self-administration at home. Various methods exist for delivering drugs and they commonly include prefilled syringes, autoinjectors, infusion pumps and, more recently, on-body delivery systems (OBDS). However, larger volumes are administered by infusion pumps or OBDS, like the enFuse®, and allow flexibility for administration at home or in the clinic.
These methods vary in complexity of development due to certain requirements with container and formulation stability. This article focuses on the impact of formulation changes and container closure systems on development time, as well as touching on patient preference.
“Larger volumes are administered by infusion pumps or OBDS, like the enFuse®, and allow flexibility for administration at home or in the clinic.”
ORIGINAL CONTAINER CLOSURE CHALLENGES WITH OBDS
Few OBDS possess the ability to use an original container closure. For OBDS that require a change in container closure to deliver the drug, drug compatibility and stability must be tested with the primary packaging material, which is often a vial or a prefilled cartridge. If the drug manufacturer chooses an OBDS that does not use the original container closure, then the drug will likely be stored in a prefilled cartridge, which qualifies as new primary packaging material. As a result, stability and compatibility testing must be performed for OBDS that store the drug within a cartridge.
Stability and compatibility testing can take several months to complete. Some tests that must be done include, but are not limited to, compendia assessment, critical quality attributes, biocompatibility testing, container closure integrity, extractables/leachables testing and particle analysis. These tests on the new primary packaging material with an OBDS that does not use the original container closure may lead to additional costs and development time, potentially prolonging the launch of the combination product.
FORMULATION CHALLENGES
High-concentration formulations are sought after by drug manufacturers for SC drug delivery. They are pursued because a higher concentration formulation can lead to a reduced total drug volume, which can potentially be administered via an autoinjector. Unfortunately, this is not always possible due to the complexity of biologic drug formulations. Several elements must be considered when concentrating biologic drugs, including but not limited to, stability, subvisible particles, viscosity, pH, osmolality and tonicity.
Ideally, a stable formulation with low viscosity and less protein aggregation is desirable; unfortunately, high-concentration formulations tend to have a high viscosity and risk of protein-protein interactions. The process of concentrating an IV dose to a small volume SC dose with proper stability and viscosity can take many months or years, depending on the molecule. Formulation choices impact many processes, including stability, manufacturability and administration.
With large-volume SC delivery, the development and formulation process could be less strenuous, depending on the situation. If a manufacturer must concentrate a high-volume IV dose to a 3 mL SC dose, the development and formulation process is likely to be more challenging and time consuming than concentrating the same dose down to 15 mL. This enables the possibility of a low-concentration, high-volume formulation, which is likely to have lower viscosity and protein-protein interactions.
“The enFuse® allows administration for a range of volumes, from 5–25 mL, with a single device.”
IMPROVEMENTS IN LARGE-VOLUME SC DRUG DELIVERY DEVELOPMENT
Enable Injections’ novel enFuse® drug delivery system delivers high-volume therapeutics through SC administration, offering a more flexible alternative to IV administration and SC options, such as an infusion pump.
The enFuse® is designed to be a discreet, lightweight and simple device that allows patients to self-administer their medication when and where it is needed. The enFuse® could alleviate the challenges pharma companies face when considering large SC delivery options by accommodating a range of drug viscosities and volumes while using the original container closure. The original container closure and the high-volume capacity could reduce development time for pharma when converting drugs from IV to SC or from SC with a pump to SC with an OBDS.

Figure 1: The enFuse® is a novel, wearable drug delivery system that can deliver large volumes of large- and small-molecule therapeutics from 5–25 mL.
Original Container Closure
The enFuse® platform allows the manufacturer to use the original container closure, allowing the potential to leverage historical data and potentially reduce time to market. Because the enFuse® uses the original container closure and does not require switching configurations to a prefilled cartridge, manufacturers can maintain their procedures and processes with the same standard vial. Depending upon the situation, by choosing large-volume SC delivery with enFuse®, drug manufacturers may be able to forgo additional validation studies, stability testing and the associated costs and time needed by other wearable injectors requiring a configuration change (Figure 1).
Range of Volume and Patient Preference
The ability to handle volumes up to 25 mL at a range of viscosities in a single device can alleviate concerns around the formulation and stability. This is because concentrating a large IV drug volume down to 10 mL or less for SC administration can be a challenge even with a permeation enhancer. In addition, the volume can impact the number of devices needed to administer the required dose (Figure 2).
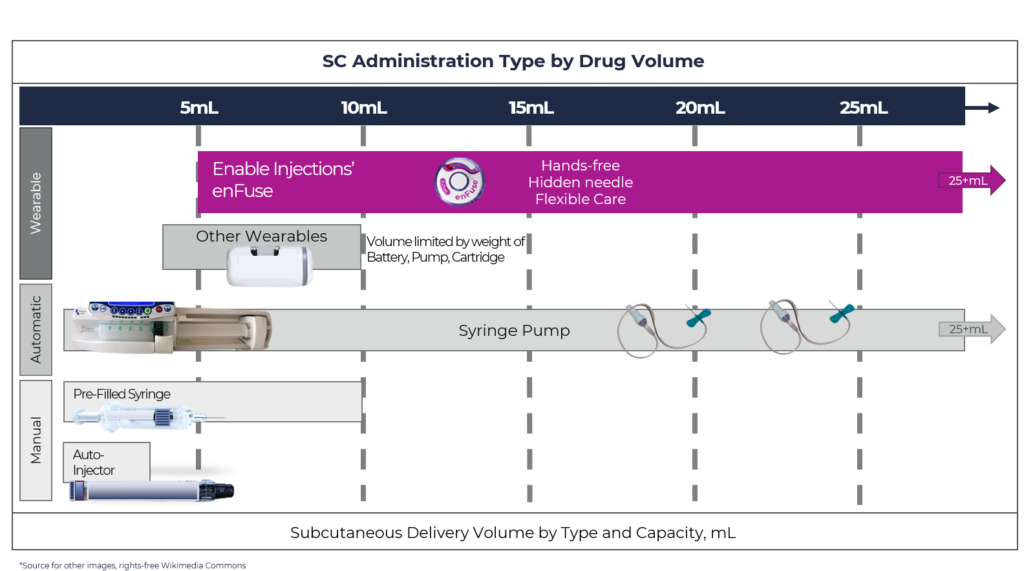
Figure 2: Overview of SC drug delivery system technology by delivery volume capacity, by type of administration for manual systems and wearable systems.
Most wearable injectors can only accommodate 3.5–10 mL, and for larger volumes, the technology becomes burdensome because two or more devices must be worn. The recent example with SC Ultomiris (ravulizumab) (Alexion Pharmaceuticals) approved for paroxysmal nocturnal haemoglobinuria (PNH) is an example of this. Ultomiris was approved as a once-weekly treatment using West Pharmaceutical Services’ SmartDose platform, specifically the SmartDose 3.5 mL OBDS. With its 3.5 mL capacity and the total weekly dose for patients >40 kg of 490 mg (7 mL), two SmartDose devices must be used per dose every week.7
In contrast, the enFuse® allows administration for a range of volumes, from 5–25 mL, with a single device. In the situation that a dose increase would be needed, for example, from 10–20 mL, the enFuse® would still only require a single device and no change would be necessary. Unfortunately, other wearable injectors would require multiple devices. Using a high-volume OBDS, such as the enFuse®, may allow a direct conversion from an IV to a SC formulation, eliminating the task of up concentrating.

Figure 3: Throughout the enFuse® injection, the needle is hidden from the user and allows for a hands-free injection.
When it comes to wearing multiple enFuse® devices or using an infusion pump, a recent study demonstrated patient preference for wearing multiple enFuse® devices rather than using an infusion pump at a mean administration volume >50 mL using SC immunoglobulin.8 The main reasons that patients preferred the enFuse® included ease of use, more mobility during infusion, less time to set up the devices and less pain at the injection site.8 The number of devices used per weekly dose ranged from two to five devices, with one patient administering almost 100 mL subcutaneously; additionally, a maximum of four devices could be used simultaneously (Figure 3).8
CONCLUSION: REDUCE PHARMA DEVELOPMENT TIME WITH THE ENFUSE®
Innovative large-volume SC delivery methods, such as the enFuse®, can accommodate various scenarios when it comes to drug development and commercialisation, for example, converting directly from IV to SC without major formulation changes. The ability to administer 5–25 mL in a single device at home or in the clinic with an original container closure has the potential to provide manufacturers with development time savings, patient preference and a faster time to market over other OBDS.
REFERENCES
- Datta-Mannan A et al, “Influence of physiochemical properties on the subcutaneous absorption and bioavailability of monoclonal antibodies. MAbs, 2020, Vol 12(1), 1770028.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- US Package Insert, Herceptin Hylecta (trastuzumab and hyaluronidase-oysk), US FDA Web Page, 2019. (https://www. accessdata.fda.gov/drugsatfda_docs/label/2019/761106s000lbl. pdf, accessed Jan 2023.)
- US Package Insert, Rituxan Hycela (rituximab and hyaluronidase human), US FDA Web Page, 2017. (https://www.accessdata.fda.gov/drugsatfda_docs/ label/2017/761064s000lbl.pdf, accessed Jan 2023.)
- US Package Insert, Darzalex Faspro (daratumumab and hyaluronidase-fihj) US FDA Web Page, 2020. (https://www.accessdata.fda.gov/drugsatfda_docs/ label/2020/761145s000lbl.pdf, accessed Jan 2023.)
- US Package Insert, Perjeta (pertuzumab), US FDA Web Page, 2012. (https://www.accessdata.fda.gov/drugsatfda_docs/ label/2012/125409lbl.pdf, accessed Jan 2023.)
- US Package Insert, Ultomiris (ravulizumab-cwvz), US FDA Web Page, 2018. (https://alexion.com/documents/ ultomiris_uspi, accessed Jan 2023.)
- Wasserman et al, “Systemic IGG exposure and safety in patients with primary immunodeficiency: A randomized crossover study comparing a novel Investigational Wearable Infusor versus the Crono Pump”. Immunotherapy, 2022, Vol 14(16), pp 1315–1328.

