To Issue 143
Citation: Saabye R, Jacobsen M, “MistGo® – A New Microdosing Eye Medication Delivery System”. ONdrugDelivery, Issue 143 (Mar 2023), pp 18–22.
Rie Saabye and Morten Jacobsen discuss the benefits of a new microdosing eye medication delivery system for chronic ophthalmic diseases, such as dry eye disease and glaucoma.
“A patient may purport to be adherent but actually be using a medication incorrectly or not taking any medication due to difficulty self-administering drops.”
According to the WHO, eye conditions are remarkably common, with 2.2 billion people having a distance or near presenting vision impairment globally, and population growth and ageing are expected to increase the risk of more people acquiring a vision impairment. Vision impairment affects people of all ages, with the majority being over the age of 50. Young children with early onset severe vision impairment can experience lower levels of educational achievement, while in adults it often affects quality of life through lower productivity, decreased workforce participation and high rates of depression.1
Of the various routes to administer drugs to the eye to treat chronic diseases, such as dry eye disease (DED) and glaucoma, the topical route is the most commonly used. Proper administration and strict patient adherence to the prescribed therapeutic regimen are important to ensure safe and efficacious use of topical ocular therapies and to preserve vision for patients with chronic conditions.2–5 On the other hand, improper instillation techniques can lead to treatment failure, adverse events and a potential risk of serious infectious complications.6–10
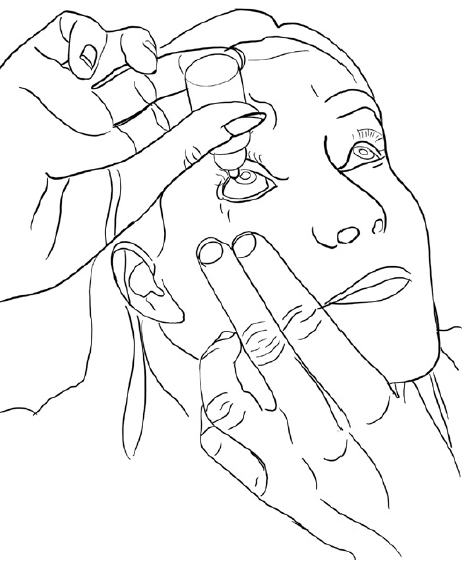
Figure 1: Use of dropper bottle.
However, many patients struggle with medication adherence; systematic reviews estimate medication non-adherence rates can be as high as 80%.11 Additionally, a patient may purport to be adherent but actually be using a medication incorrectly or not taking any medication due to difficulty self-administering drops. Numerous studies in the US and other countries have documented that poor technique is a considerable concern in non-adherence.12
The way topical medication is administered by patients has not changed much in a century. Patients are still relying on dropper bottles, trying to instil just a single drop into the eye with the head in awkward positions, as illustrated in Figure 1.
On top of that, even if the patient successfully instils just one drop from a conventional eyedropper – delivering highly variable 30–50 μL drops that greatly exceed the physiologic 6-8 μL ocular tear film capacity13,14,15 – the overdosing floods the eye with excess drug compounds and preservatives, resulting in ocular surface and systemic toxicity.16,17 Collectively, these findings emphasise the need for an improved delivery technology for topical ophthalmic therapies.
MISTGO®: THE NEXT-GENERATION DELIVERY SYSTEM
“MistGo® is designed with the user in mind to ensure a successful instillation every time.”
The Danish MedTech company EYE-GO has developed a new-generation microdosing delivery system for topical ophthalmic therapies, eliminating the well-known barriers of conventional eyedroppers. MistGo®, shown in Figure 2, is designed with the user in mind to ensure a successful instillation every time. It releases a gentle optimal mist dose of down to 6 μL evenly and precisely across the cornea of the eye for optimal drug delivery. It is compatible with multiple atomisable substances and hence useful for many chronic eye diseases. The aim of MistGo® is to let the topical ophthalmic therapy perform at its best with adherent patients enjoying the full treatment benefits without exposure to unnecessary side effects.

Figure 2: The MistGo® microdosing delivery system.
EASE OF USE
Evaluations of eye-drop self-administration show that 34–92% of patients use improper instillation techniques,5,9,10,18–22 7–44% miss the eye completely,9,18,20-21,23,24 and 18–80% contaminate the tip of the bottle by contacting the eye or surrounding tissue.1,7–10,18,20–27 Additionally, up to 30% of patients instil a stream of fluid rather than the prescribed number of drops, resulting in excessive delivery and increasing the risk of local and systemic adverse events.21
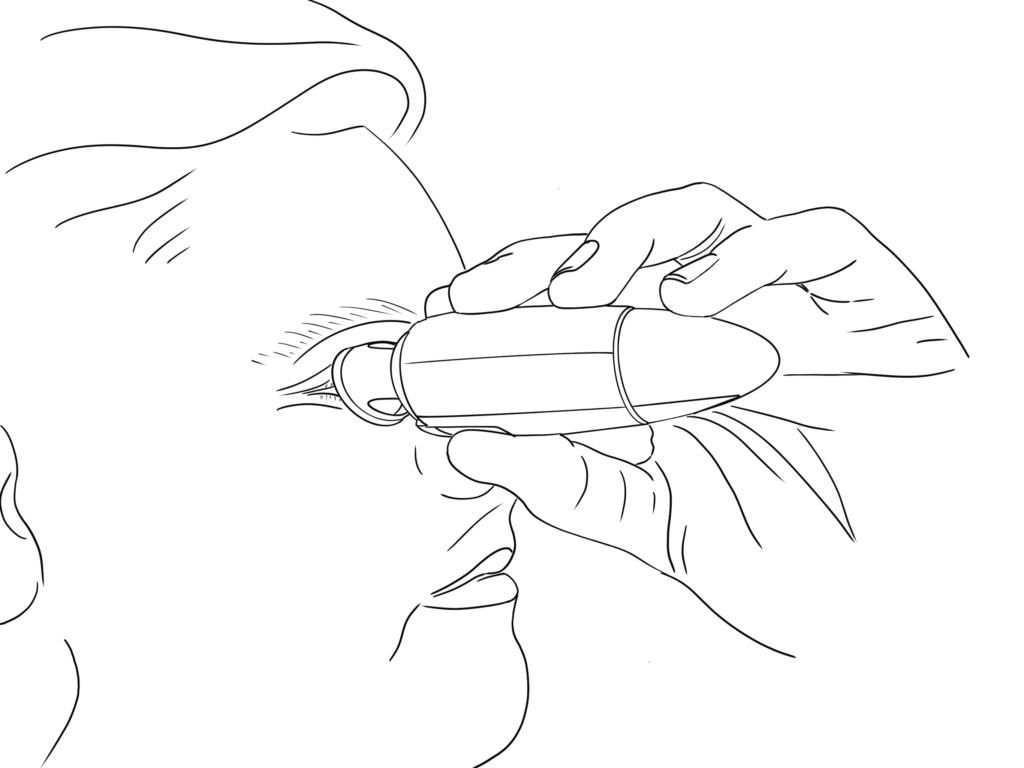
Figure 3: User-friendly design.
Adherence status has been found to be statistically associated with the instillation technique of topical glaucoma medications. Adherence and instillation proficiencies are interconnected, meaning that poor practice in instillation could jeopardise adherence and vice versa.5 MistGo® is intuitive, easy and comfortable to use, even for seniors and children, and creates a better user experience, which translates into higher patient compliance, healthier eyes and higher quality of life for patients.
MistGo® is omnidirectional – it can be used horizontally, as depicted in Figure 3, or in any head position preferred. It has an integrated eye interface to be placed around the eye socket to ensure a safe topical administration precisely to the cornea without having to tilt the head in an unnatural position and without accidentally touching the eye with the device.
In a 2019 usability study conducted by an independent institute with a sample of participants above the age of 50, MistGo® was rated 8.44 when asked “on a scale from 1–10, how would you rate the user friendliness of the product?” and 9.94 when asked “on a scale from 1–10, how confident would you feel to dispense a mist to the eye with the product at home?”
Visit EYE-GO’s website for an animation of how MistGo® is used.
PRESERVATIVE FREE
Eye drops delivered in a multidose container must maintain the sterility of the contents throughout the intended lifespan of the product. Antimicrobial activity is often achieved through the addition of preservatives. The most frequently used preservative, benzalkonium chloride (BAK), has consistently demonstrated its toxic effects in laboratory, experimental and clinical studies.28
Whilst BAK is known to be an effective antimicrobial agent, demonstrating efficacy against a wide variety of common pathogens, considerable evidence, often from its use in glaucoma medications, also exists detailing the deleterious effects it has on the ocular surface, particularly when used over an extended period of time. It can be argued that the undesirable effects of BAK have contributed to a movement into preservative-free topical preparations. Removal of the preservative naturally eliminates preservative-induced complications. In both glaucomatous and DED populations, use of preservative-free drops can improve signs and symptoms compared with BAK-containing formulations.29
A preservative-free eye medication must be dispensed in either a single-use vial or delivery systems specifically designed to keep the liquid formulation sterile throughout the treatment period. MistGo® is airtight and designed to maintain sterility in the following ways:
- MistGo® integrates a collapsible multilayer container (the cartridge), obviating the need to ventilate with contaminated air during multiple dosage. Therefore, there is no back flow of air that requires filtering.
- The liquid is sealed off from the environment using non-permeable material. Several barriers are further established to separatee the liquid from the environment, including a one-way valve.
- The system enables pressurised air to both create the mist and completely clean the outlet nozzle chamber after dispensing. This leaves no liquid for later microbiological contamination. The liquid column is broken and contamination is prevented.
- An eye interface protects the nozzle from contamination caused by contact with the user, and a lid helps protect the dosing chamber from contamination during storage between uses.
“Reducing the drop volume avoids problems with overflow and may increase cornea absorption by reducing lacrimal drug dilution and tear loss due to blinking and drainage.”
UNIQUE MICRODOSING CONTROL
The cornea is the main route for topically applied drugs to gain access into the eye, although the conjunctival/scleral route can also be efficient. However, efficient ocular drug delivery is hampered by a series of clearance mechanisms protecting the ocular structures from foreign matter, such as reflexive tearing or blinking, nasolacrimal drainage increasing precorneal drug dilution and clearance from the eye.30
The tear film covering the ocular surface is approximately 3–10 μL in volume, 3 μm thick and secreted at a rate of 1–2 μL/min.31 Commercial eye drops vary significantly between 25 and 70 μL. Typically, after instillation of an eye-drop, less than 5% of the applied drug penetrates the cornea and reaches the intraocular tissues; the major fraction of the instilled drug is absorbed and enters systemic circulation, risking adverse effects. A smaller eye drop can achieve a maximal tear film concentration with far less systemic absorption. Hence, from a biopharmaceutical and economic point of view, the ideal would be to instill smaller volumes of 5–15 μL.32,33
Reducing the drop volume avoids the previously mentioned problems with overflow and may increase corneal absorption by reducing lacrimal drug dilution and tear loss due to blinking and drainage.34 MistGo® contains a high-precision micropump, consistently metering a dose of 6 μL, dispensing no excess liquid that can irritate the skin or enter systemic circulation, risking adverse events. Compressed air is used to propel the mist out of the device at just the right velocity for the full dose to reach the cornea safely and comfortably before the eye can blink. The internal geometry of the nozzle is designed to vaporise the drug into a fine mist with a broad impact pattern, coating the entire cornea for optimal absorption (~10–12 mm diameter).
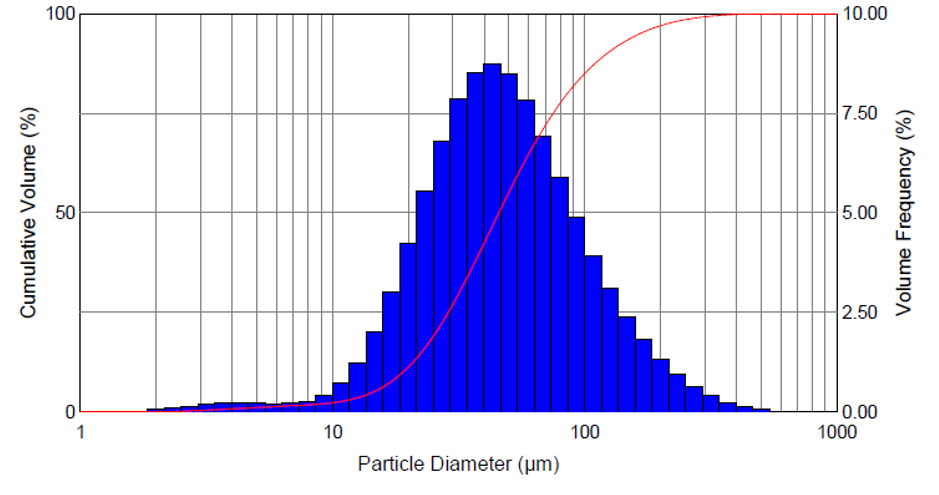
Figure 4: Droplet size distribution.
The mist contains mini droplets that vary in size, depending on viscosity, surface tension level and other characteristics of the drug. Figure 4 depicts vaporisation of the glaucoma drug latanoprost, resulting in a homogeneous distribution of droplets with a mean diameter of approximately 50 μm. Figure 5 shows misting of the glaucoma drug latanoprost with MistGo. Parameters inside MistGo® can be adjusted to suit the needs of individual drugs, enabling the delivery system to perform optimally for that specific liquid – allowing users to have an ideal experience of a soothing mist.

Figure 5: Mist of glaucoma medication.
MistGo® received high scores on perceived comfort, with no bother at all from excess fluid after treatment, at a comfort study conducted together with the Department of Ophthalmology at the University Hospital in Copenhagen (Denmark) in 2021. The sample included patients aged 40–90 suffering from DED with mild to severe symptoms. On a scale from 1–5, the average score was 4.5 when asked, “To what extent do you prefer the mist over eye drops when treating your eye?”
“The internal geometry of the nozzle is designed to vaporise the drug into a fine mist with a broad impact pattern, coating the entire cornea for optimal absorption.”
INVITATION TO PARTNER
EYE-GO is currently preparing for verification of the delivery system as well as setting up for design-for-manufacturing (DFM) for mass production. MistGo® is a delivery system that can be combined with eye medications. The US FDA and EU EMA have, over the last few years, expanded and clarified their requirements for drug-device combination products.
Under FDA regulations, therapeutic products combining a drug and a device are classified as combination products. When the drug will be the primary mode of action (PMoA), the Center for Drug Evaluation and Research (CDER) will be the primary regulatory branch evaluating and approving. Combination products are subject to 21 CFR Part 4, and the submission may follow the condensed 21 CF 4.4(b) filing, demonstrating compliance with both the drug cGMPs (21 CFR parts 210 & 211) and provisions of the device quality system regulation (21 CFR part 820).35
The EMA also regulates based on the PMoA, and if MistGo® is combined with Rx eye medication it will be defined as “medicinal product used in combination with a medical device”. The EMA has published a final guideline (taking effect January 2022) of the expanded requirements, including elements such as Annex 1 of MDR 2017/745.36,37 Drug manufacturers are required to submit a notified body opinion to the EMA confirming that the device meets the General Safety and Performance Requirements of MDR 2017/745 Annex 1 requiring data on risk management, safety, performance, functionality, usability, sterilisation validation and microbiology.38
With these increasing demands and stricter regulatory filing requirements, pharma companies are wise to select a device partner with a high-quality delivery system and expertise to provide all the required documentation on the device. EYE-GO’s MistGo® is human-factors engineered and designed for safe, comfortable and effective use. EYE-GO’s quality management system, including risk management, will be fully compliant with the requirements of the regulatory authorities, and will support customers in all aspects possible in the application process.
EYE-GO is currently inviting pharma companies with the ambition to adopt a best-in-kind eye-drop delivery system to a dialogue about future partnerships for their substances, whether they are in the pipeline preclinical, clinical or currently on the market.
REFERENCES
- “Eye care, vision impairment and blindness”. Web Page, WHO, accessed Dec 19, 2022.
- Davis SA et al, “Drop instillation and glaucoma”. Curr Opin Ophthalmol,2018, Vol 29, pp 171–177.
- “The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators”. Am J Ophthalmol, 2000, Vol 130, pp 429–440.
- Kass MA, Heuer DK, Higginbotham EJ et al, “The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma”. Arch Ophthalmol, 2002, Vol 120, pp 701–713, 829–830.
- Atey TM et al, “The impact of adherence and instillation proficiency of topical glaucoma medications on intraocular pressure”. J Ophthalmoly, 2017, Vol 2017, Article 1683430.
- Schein OD et al, “Microbial keratitis associated with contaminated ocular medications”. Am J Ophthalmol, 1988, Vol 15, pp 361–365.
- Schein OD et al, “Microbial contamination of in-use ocular medications”. Arch Ophthalmol, 1992, Vol 110, pp 82–85.
- Geyer O et al, “Microbial contamination of medications used to treat glaucoma”. Br J Ophthalmol, 1995, Vol 79, pp 376–379.
- Sleath B, Blalock S, Covert D et al, “The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity”. Ophthalmology, 2011, Vol 118, pp 2398–2402.
- Gupta R et al, “Evaluating eye drop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21, pp 189–192.
- Newman-Casey PA et al, “Patterns of Glaucoma Medication Adherence over Four Years of Follow-Up”. Ophthalmology, 2015, Vol 122(10), pp 2010–2021.
- Robin A, Muir K, “Medication adherence in patients with ocular hypertension or glaucoma”. Expert Rev Ophthalmol, 2019, Vol 14(4-5), pp 199–210.
- Washington N, Washington C, Wilson CG, “Ocular drug delivery”. Physiological Pharmaceutics: Barriers to Drug Absorption, 2nd edition, CRC Press, 2001, pp 249–270.
- Mishima S et al, “Determination of tear volume and tear flow”. Invest Ophthalmol, 1966, Vol 5(3), pp 264–276.
- Scherz W, Doane MG, Dohlman CH, “Tear volume in normal eyes and keratoconjunctivitis sicca”. Albrecht Von Graefes Arch Klin Exp Ophthalmol, 1974, Vol 192(2), pp 141–150.
- Izazola-Conde C, Zamora-de La Cruz D, Tenorio-Guajardo G, “Ocular and systemic adverse effects of ophthalmic and non ophthalmic medications”. Proc West Pharmacol Soc, 2011, Vol 54, pp 69–72.
- Quaranta L, Gandolfo F, Turano R et al, “Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma”. Invest Ophthalmol Vis Sci, 2006, Vol 47(7), pp 2917–2923.
- Tatham AJ et al, “Eye drop instillation technique in patients with glaucoma”. Eye, 2013, Vol 27 pp 1293–1298.
- Konstas AG et al, “Compliance and viewpoint of glaucoma patients in Greece”. Eye, 2000, Vol 14, pp 752–756.
- Kholdebarin R et al, “Multicenter study of compliance and drop administration in glaucoma”. Can J Ophthalmology, 2008, Vol 43, pp 454–461.
- Stone JL et al, “An objective evaluation of eyedrop instillation in patients with glaucoma”. Arch Ophthalmol, 2009, Vol 127, pp 732–736.
- Mehari T, Giorgis AT, Shibeshi W, “Appropriateness and determinants of proper administration technique of ocular hypotensive agents among glaucoma patients in Menelik II referral hospital, Ethiopia”. J Clin Exp Ophthalmol, 2016, Vol 7(3).
- Hennessy AL, Katz J, Covert D et al, “A video study of drop instillation in both glaucoma and retina patients with visual impairment”. Am J Ophthalmol, 2011, Vol 152, pp 982–998.
- Schwartz GF, Hollander DA, Williams JM, “Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension”. Curr Med Res Opin, 2013, Vol 29, pp 1515–1522.
- Brown MM, Brown GC, Spaeth GL, “Improper topical self-administration of ocular medication among patients with glaucoma”. Can J Ophthalmol, 1984, Vol 19, pp 2–5.
- Hosoda M et al, “Do glaucoma patients use eye drops correctly?” J Glaucoma, 1995, Vol 4, pp 202–206.
- Tsai T, Robin AL, Smith JP 3rd, “An evaluation of how glaucoma patients use topical medications: a pilot study”. Trans Am Ophthalmol Soc, 2007, Vol 105, pp 29–33.
- Baudouin C et al, “Preservatives in eyedrops: The good, the bad and the ugly”. Progress in Retinal and Eye Research, 2010, Vol 29(4), pp 312-334.
- Walsh K, Jones L, “The use of preservatives in dry eye drops”. Clin Ophthalmol. 2019, Vol 1(13), pp 1409-1425.
- Morrison PW, Khutoryanskiy VV, “Advances in ophthalmic drug delivery”. Ther Deliv, 2014, Vol 5(12), pp 1297–1315.
- Chang AY, Purt B, “Biochemistry, Tear Film”. In: StatPearls, Jun 11, 2022.
- Van Santvliet L, Ludwig A, “Determinants of eye drop size”. Surv Ophthalmol, 2004, Vol 49(2), pp 197-213.
- Brown RH, Hotchkiss ML, Davis EB, “Creating Smaller Eyedrops by Reducing Eyedropper Tip Dimensions”. Am JOphthalmol, 1985, Vol 99(4), pp 460-464.
- Martini LG et al, “The use of small volume ocular sprays to improve the bioavailability of topically applied ophthalmic drugs”. Eur J Pharm Biopharm, 1997, Vol 44(2), pp 121-126.
- “Guidance for Industry and FDA Staff: Current Good Manufacturing Practice Requirements for Combination Products – FINAL GUIDANCE”. US FDA, 2015.
- “Guideline on quality documentation for medicinal products when used with a medical device – First version”. EMA, 2021.
- “Regulation (EU) 2017/745 of the European Parliament and of the council”. European Parliament and the Council, Apr 5, 2017.
- “MDR Article 117: Implications for Drug-device Combination Products”. Company Web Page, Celegence, accessed Dec 12, 2022.

