To Issue 156
Citation: Hasegawa H, Suzuki T, “Container Closure Integrity of OXYCAPT™ Vial at -80°C with Dry Ice”. ONdrugDelivery, Issue 156 (Jan 2024), pp 88–92.
Hiroki Hasegawa and Tomohiro Suzuki highlight the overall benefits of OXYCAPT, Mitsubishi Gas Chemical’s multilayer plastic vial, as a primary container for biologics and gene and cell therapies and discuss the company’s recent investigation into OXYCAPT’s performance at -80°C under dry ice conditions.
OXYCAPT™ OVERVIEW

Figure 1: OXYCAPT multilayer plastic vial.
OXYCAPT™ is a multilayer plastic vial developed by Mitsubishi Gas Chemical (MGC) that offers a number of advantageous qualities as a primary drug container (Figure 1). The material consists of three layers – the drug contact layer and the outer layer are made of cyclo-olefin polymer (COP), and the oxygen barrier layer is made of MGC’s novel polyester (Figure 2). MGC continuously conducts tests to confirm OXYCAPT’s excellent properties, including:
- Excellent oxygen and ultraviolet (UV) light barrier
- Strong water vapour barrier
- Very low extractables
- High pH stability
- Low protein adsorption and aggregation
- High transparency
- High break resistance
- Easy disposability
- Lightweight material.
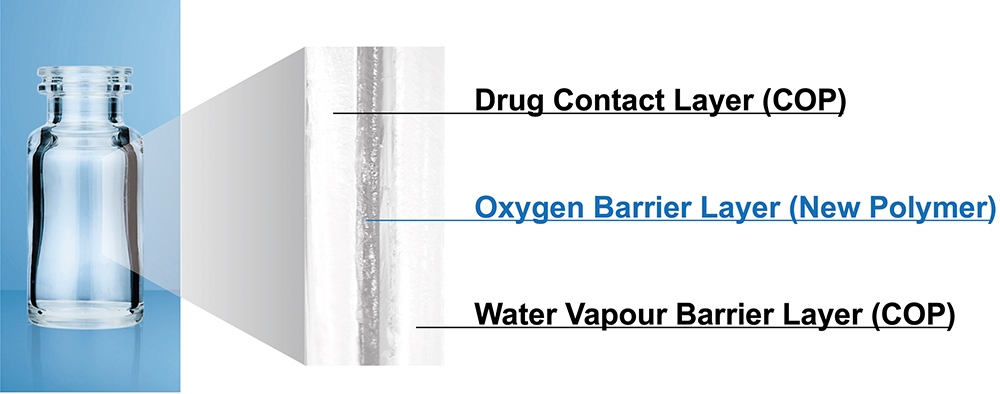
Figure 2: Multilayer structure of OXYCAPT.
“Studies have shown an extremely low level of extractables from OXYCAPT.”
MGC recently obtained a report on the environmental impact of glass and plastic containers for medical use from a Japanese research company. The report shows that plastic containers for medical use are much more environmentally friendly compared with glass containers. For example, the carbon footprint, nitrogen oxides emissions, sulfur oxides emissions and water consumption associated with plastic containers for medical use are several times smaller than those of their glass equivalents.
OXYCAPT provides an excellent oxygen barrier. For example, the oxygen barrier of an OXYCAPT vial is about 20 times better than that of a COP monolayer vial. Furthermore, OXYCAPT provides an excellent UV barrier. While about 70% of 300 nm UV light transmits through glass and COP, only 1.7% transmits through OXYCAPT. MGC has confirmed that this feature contributes to the stability of biologics.
While OXYCAPT cannot reach the performance of glass with respect to acting as a water vapour barrier, its properties are similar to those of COP, which has been used for injectable drugs for a long time. This means that OXYCAPT easily meets the requirements of a water vapour barrier set out by the ICH guidelines.
Studies have shown an extremely low level of extractables from OXYCAPT. One study was conducted to confirm the levels of volatile, semi-volatile and non-volatile impurities from OXYCAPT. Water and four solutions (50% ethanol, NaCl, NaOH and H3PO4) were selected, and impurities were measured by gas chromatography mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with the control, impurities were not detected in the OXYCAPT containers. A second study confirmed that inorganic extractables levels from OXYCAPT were similar to those from COP, which is well known for being an extremely pure polymer with a better extractables profile than Type 1 glass. Lower levels of inorganic extractables are known to contribute to better pH stability in drug products.
The OXYCAPT vial is produced by co-injection blow-moulding technology. MGC has also developed inspection methods for testing the oxygen barrier layer. All the containers are fully inspected by state-of-the-art inspection machinery.
“During storage and transportation, packages, including vials, are exposed to temperatures of around -80°C in a deep freezer or dry ice, which is a potential risk to CCI due to differences in the CTE of the vial and rubber closure materials.”
MGC can offer bulk vials and ready-to-use (RTU) vials, with its RTU products provided in standard nest and tub or tray formats. The nest and tub are mainly sterilised using gamma rays. There are 2, 6, 10 and 20 mL variants for vials. MGC is willing to provide samples for initial testing free of charge.
Each polymer meets the requirements of US Pharmacopeia (USP) regulations USP <661>, USP <87> and USP <88>, as well as those of the European Pharmacopoeia, and has been filed in the US FDA’s drug master file (DMF). The vials are also compliant with each pharmacopoeia and have been filed in the DMF.
The primary target market for OXYCAPT is the therapeutic application of biologics. As mentioned in ICH Q5C (Stability of Biotechnological/Biological Products), oxidation is one of the causes of protein instability. As such, the oxygen and UV barrier properties of OXYCAPT will definitely contribute to the stability of biologics stored within. Furthermore, some drug developers have recently started evaluating the OXYCAPT vials for their gene and cell therapies; the RTU vial is sterilised by gamma radiation, making it ideal for protein-based drugs.
CONTAINER CLOSURE INTEGRITY AT -80°C
All pharmaceutical containers must maintain integrity against microbial contamination and have a gas barrier when a drug is sensitive to oxygen or carbon dioxide (CO2). Figure 3 shows a typical scheme of storage and transportation for gene therapy. During storage and transportation, packages, including vials, are exposed to temperatures of around -80°C in a deep freezer or dry ice, which is a potential risk to container closure integrity (CCI) due to differences in the coefficient of thermal expansion (CTE) of the vial and rubber closure materials.
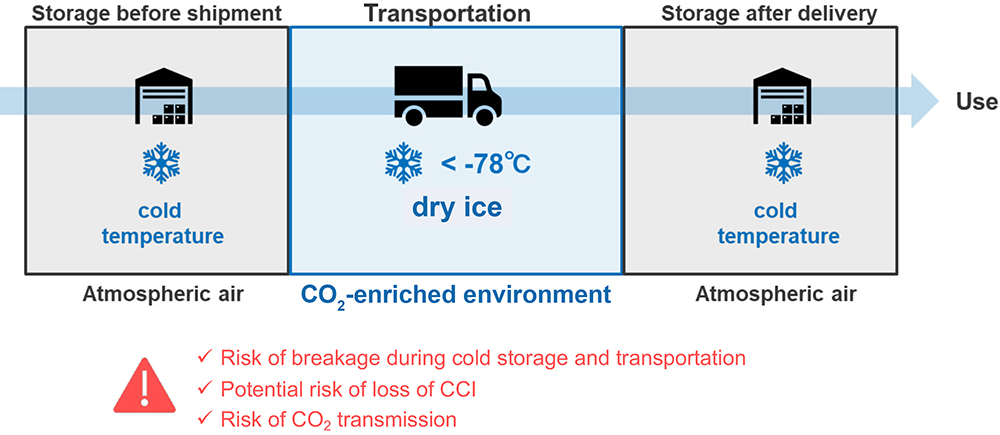
Figure 3: Typical scheme of storage and transportation for gene therapy.
“Standard plastic vials have a potential risk of CO2 transmission when in storage with dry ice.”
The CCI of Type I glass vials is particularly at risk from very low temperature compared with plastic vials because the CTE of typical Type I glass is a factor of 10 smaller than that of rubber, including a halogenated butyl rubber. On the other hand, standard plastic vials have a potential risk of CO2 transmission when in storage with dry ice. Based on MGC’s calculation by measurement of the transmission rate of CO2 through a polymer film, OXYCAPT has a CO2 barrier more than four times better than comparable COP monolayer vials. This means that OXYCAPT vial has the potential to significantly contribute to protecting drugs, including biologics and gene and cell therapies, when they are in transport with dry ice.
To examine this potential benefit further, MGC performed a CCI test with dry ice. Table 1 shows the test sample combinations of OXYCAPT vial and rubber closures. Rubber Closure 1 is a typical closure made of bromo butyl rubber with a glass transition temperature of -65°C. MGC also prepared press-on-cap closures and OXYCAPT’s positive control with a fine hole of a 5 μm nominal diameter.
| Entry | Vial configuration |
Vial | Rubber Closure | Aluminium seal cap |
| 1 | OXYCAPT/Rubber closure 1 | OXYCAPT-P 10 mL Vial | Bromo butyl rubber | Standard one with closure 1 |
| 2 | OXYCAPT/Press-on-cap closure 2 | OXYCAPT-P 10 mL Vial | Press-on-cap closure | |
| 3 | OXYCAPT/Rubber closure 1/Positive control | OXYCAPT-P 10 mL Vial | Bromo butyl rubber | Standard one with closure 1 |
Table 1: Test sample combinations of OXYCAPT vial and rubber closures.
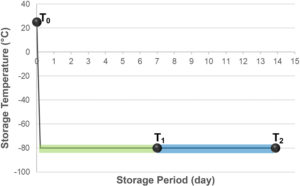
Figure 4: Test procedure of storage in deep freezer and insulation box with dry ice.
Figure 4 shows the test procedure, which includes storage in a deep freezer and an insulation box with dry ice. First, all the vials, closures and aluminium seals were inserted into a chamber where the air was replaced with nitrogen, then they were assembled by hand in the chamber. After preparing the samples, MGC measured the partial pressure of CO2 in the vials’ headspace for all the samples (T0). The samples were then stored in a deep freezer at -80°C for seven days. After storage in the freezer, the CO2 pressure of the headspace was measured (T1). Next, the remaining samples were immediately inserted into an insulation box that was filled with 30 kg of dry ice, as shown at Figure 5. After storage in the CO2-enriched environment, CO2 pressure in the headspace was measured (T2).

Figure 5: Dry-ice blocks in insulation box.
“CO2 ingress was not be observed in either combination of OXYCAPT and the two types of closure, even at T2.”
Headspace pressure of CO2 was measured with an FMS-Carbon Dioxide, manufactured by LIGHTHOUSE Instruments (VA, US).The instrument is based on frequency modulation spectroscopy (FMS), which is a non-destructive method. Table 2 shows the sample number for each measurement time point. The measured vials were disposed of after the measurements at T1 and the remaining ones were measured at T2.
| Entry | Vial | Stopper | T0 The number of all samples |
T1 (7 days) |
T2 (7 + 7 days) |
| 1 | OXYCAPT | Rubber closure 1 | 40 | 20 | 20 |
| 2 | OXYCAPT | Press-on-cap closure 2 | 40 | 20 | 20 |
| 1’ | OXYCAPT, Positive control | Rubber closure 1 | 10 | 5 | 5 |
Table 2: Sample number for each measurement time point.
At temperatures lower than -65°C, bromo butyl rubber loses its elastic properties, which may lead to loss of airtightness at the interface between vial and rubber closure. Therefore, maintaining a temperature inside the insulation box of under -65°C is crucial for measuring the leakage precisely in this test. Figure 6 shows a temperature log inside the insulation box during the test, which was kept below -70°C for seven days.
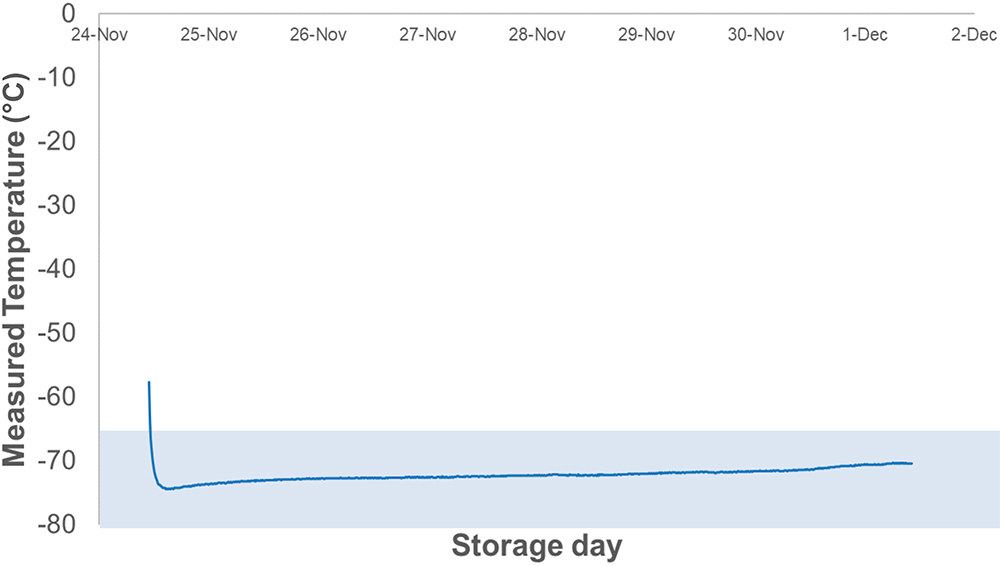
Figure 6: Logging temperature data in the insulation box.
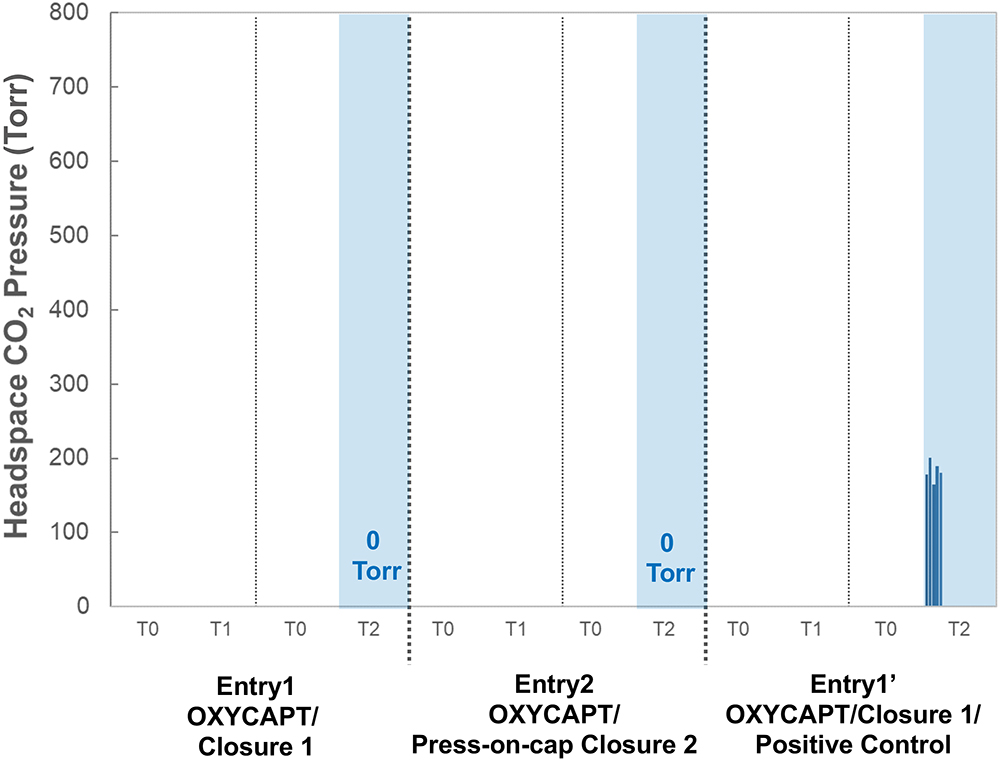
Figure 7: Headspace CO2-enriched pressure for Entry 1, 2 and 1’.
Figure 7 shows the results of headspace CO2 pressure for Entries 1, 2 and 1′. Regarding OXYCAPT positive control of Entry 1’, the mean value of CO2 pressure was 183 Torr at T2 under a CO2-enriched environment. However, there was no CO2 ingress at T1, as the initial seven-day storage was conducted under atmospheric conditions without dry ice. On the other hand, CO2 ingress was not observed in either combination of OXYCAPT and the two types of closure (Entry1 and Entry2), even at T2. This study demonstrated that OXYCAPT has an excellent CCI under a CO2-enriched environment for seven days.
CONCLUSION
There are several factors that can affect CCI for a combination of vials and closures, including capping force and type of closure, among others. In addition, CO2 transmission is potentially observed in long-term storage with dry ice and an increase in temperature during storage. MGC intends to devise and perform additional CCI tests to clarify the efficiency of OXYCAPT vials compared with other plastic and glass vials. Furthermore, MGC is also planning to conduct similar studies at -180°C to confirm the effectiveness for gene and cell therapies.
These latest results have contributed to the ongoing studies verifying OXYCAPT’s superior properties for biologics and gene and cell therapies. In addition to the advantages of COP, such as a strong water vapour barrier, high break resistance, very low extractables and low protein adsorption, OXYCAPT also provides strong oxygen and UV light barriers. MGC believes that OXYCAPT offers a multitude of benefits to the rapidly growing field of biologics and gene and cell therapies.
Previous article
PRACTICAL PATHWAYS TO SUSTAINABILITYNext article
DELIVERY SOLUTIONS FOR DEEP-COLD DRUGS
