To Issue 158
Citation: Assia S, “Human Factors in the Absence of a Familiar Mental Model: Challenges and Opportunities in Self-Administered Drug-Delivery Devices”. ONdrugDelivery, Issue 158 (Apr 2024), pp 66–70.
Shai Assia explores the intricate challenge of designing drug-device combination products for self-administration by lay users without medical expertise. Focusing on targeted nasal drug delivery devices, Mr Assia highlights the importance of overcoming user misconceptions and addresses the complexities of assessing usability when users lack prior experience with a unique mode of action.
As advancements in medical treatments continue, targeted nasal drug delivery devices are emerging as an innovative solution for various medical conditions. However, how can their practicality and user-friendliness be effectively evaluated if no one has ever experienced them before? Chances are, that even if someone has never used an inhaler or autoinjector themselves, they have at least seen one or know someone that has. But what is the point of reference for targeted deep intranasal spraying?
“From the patient’s perspective, the device constituent of the drug-device combination adds a new parameter to the treatment –
the product’s usability.”
From the patient’s perspective, the device constituent of the drug-device combination adds a new parameter to the treatment – the product’s usability. A device needs to be operated, and operated properly.
Delving deeper into the heightened complexity of self-administered devices, the target users are ordinary individuals without a medical background or extensive technical knowledge. They seek simplicity and intuitiveness in their devices, as complex interfaces could hinder their ability to adhere to prescribed treatments.
Despite these complexities, self-administration has many benefits, including putting the control in the hands of the patients, empowering them to take an active role in their own treatment and potentially improving compliance. It is available to them wherever and whenever they need it, without having to go to the clinic, wait in line or rely on someone else. All this, of course, assumes that they know how to use it.
Here lies the challenge in ensuring that the device is sufficiently foolproof to be intuitive to new users and experienced ones alike, as not all may relate to the same previously familiar mental model.
Put simply, a mental model is a description of one’s thought process relating to a particular subject. It uses our intuitive perception to associate the placement of a subject in the real world and relate it to familiar surroundings. Note the key word “perception” – mental models are based on beliefs, not facts; being subject to personal interpretation, beliefs can be misleading.
“Offering a self-administered SPG-block treatment provides patients with independence and can greatly improve their quality of life.”
CASE STUDY – CLEXIO BIOSCIENCES’ SPRACISE
Clexio Biosciences is developing SPRACISE, the device component of a drug-device delivery system for the treatment of acute cluster headaches by precise deep intranasal spraying.
On top of the challenges already mentioned, SPRACISE faced another obstacle – its indication. Cluster headache is a neurological disorder characterised by recurrent severe headaches. Cluster headaches often go undiagnosed, as most of the general population is unfamiliar with the condition, which affects about 0.1% of people worldwide, despite being referred to as the “most severe pain known to man”.
Clexio’s SPRACISE targets a specific nerve pathway at the sphenopalatine ganglion (SPG), as shown in Figure 1. While such neural modulation has been performed for over 100 years,1 to date, the procedures have only been performed by trained healthcare professionals (HCPs). Due to the explosive nature of cluster headache attacks and the frequency of the attacks during the cluster period, it is reasonable for patients to receive the treatment they desperately need outside of the clinic and right at the onset of an attack.
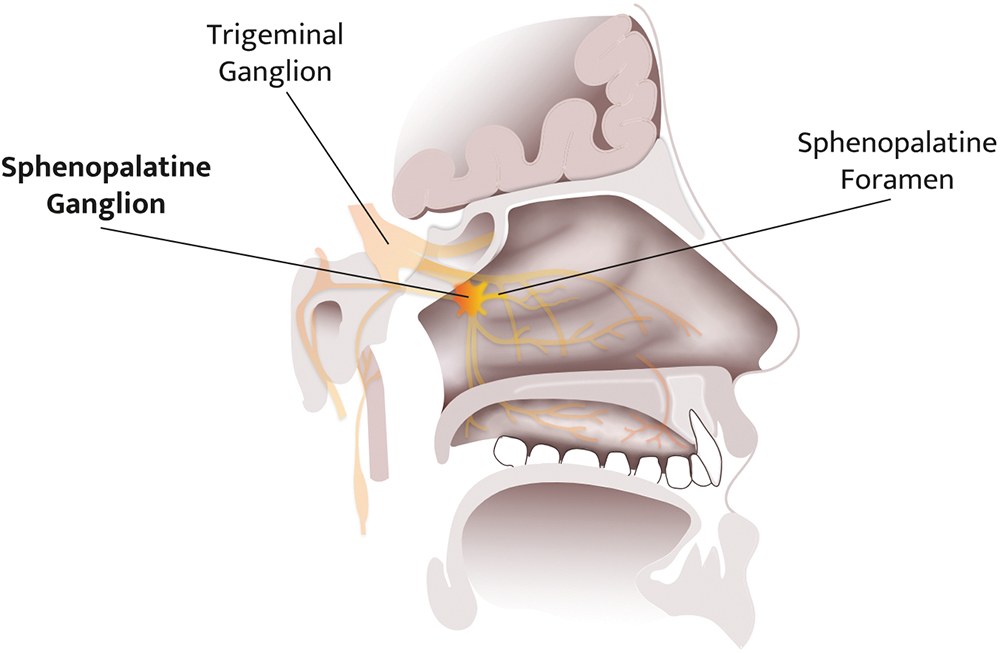
Figure 1: Illustration of the SPG’s location in the deep intranasal cavity.
Since the treatment is used as a relief medication, it must be within reach at all times. Offering a self-administered SPG-block treatment provides patients with independence and can greatly improve their quality of life.
This intranasal device involves a new and unusual use case for which lay users have no previous experience, leaving them to make up their own set of beliefs about the nasal environment. It is therefore not just the lack of any familiar mental model that makes the design challenging, but also the need to overcome existing incorrect mental models. For instance, many people perceive the nose as going upwards rather than backwards, making patient education crucial to ensure proper usability.
An interesting example is the covid-19 pandemic leading to a greater awareness of nasal anatomy in respect to respiratory illnesses. Antigen tests for covid-19, which often involved a nasal swab to collect a sample from the back of the nasal cavity, may have had different effects on people’s perceptions of nasal applicators. For some, the unpleasant experience may have led to reluctancy towards nasal applicators in general. For others, the experience may have been less painful than anticipated and more acceptable.
However, that is not the only change covid-19 introduced in this context. The pandemic overwhelmed the already-busy healthcare services and made it difficult to give (or receive) in-person treatment, demonstrating just how valuable self-administration is for medication adherence. At the same time, it also created a new standard of care, giving society a nudge forward into the connected and digital era. Tech-averse patients (predominantly in the older population) became accustomed to using remote tools for daily matters. The normalisation of telemedicine, for example, has not only enabled but also created an expectation that more forms of treatment will become available for home-based care as a standard practice.
“Determining spray deposition in the nasal cavity is challenging due to anatomical variability, variations in airflow patterns and complex fluid dynamics.”
METHODS FOR IMPROVING HUMAN FACTORS
Several key points should be considered in the design and development process to improve the overall user experience and appropriate usage of a novel device.
User Interviews
The best way to learn about users’ preferences is to ask them directly. However, medical devices are not typical consumer products, and the term “users” may refer to a few different stakeholders – patients, caregivers, general practitioners or clinical experts – all of which have relevant needs. It is important to keep in mind that user experiences are subjective, and each may emphasise different product features as the most important. Clinicians who treat many patients will assist in understanding the common pain points for many patients and can assist in discerning the exception from the rule. It is therefore helpful to include a wide range of users in surveys and interviews, and group them into fields of expertise.
Interviewing users is obvious advice, but it is important to remember that common sense is not always common practice. Many people do not listen with the intention to learn, but rather with the intention to reply. Try this little trick: embrace the awkward silence! Ask a question, listen to the answer, then say nothing. Stare blankly into the eyes of the person in front as if expecting them to continue their sentence. More often than not, they will. By planning not to speak at the first opportunity, the interviewer can resist the urge to drift off and remain a focused listener. The interviewee will, in turn, fill the void by further elaborating their point. It is a win-win in the game of active listening.
Design Principles
With the information gathered and the design input requirements clearly defined, it is time to move on to the design process itself. Prioritise the wish-list of product features, with the aim of keeping it as simple as possible – more is not always better. The product attributes need to be fit for purpose, otherwise the design reaches diminishing returns.
Ergonomics are often overlooked but have a role to play in self-administered drug delivery systems. Best practices can be found online from principles of hand-held devices and grip indicators to features that provide real-time feedback of proper use. Sophistication in device designs can also mean becoming more forgiving to inevitable human error.
Formative Human Factors Studies
The direct approach to user interviewing is the simplest one, but beware of its caveats. As previously mentioned, mental models can be misleading. One might claim that they understood how to use a device (and in their mind, they do believe this to be true), when in fact they are not operating it correctly. For this reason, it is essential for a trained professional to observe users and pick-up on any non-verbal cues.
Throughout the development of SPRACISE, formative human factors studies (HFSs) were structured in different ways, depending on the main objective at each stage of development.
- Preliminary user preferences: Focusing on product presentation. The test subjects included a mixture of HCPs and patients, who were given low-fidelity mock-ups of widely different design concepts, and were asked for feedback on comfort, intuitiveness and level of trust.
- Design concepts down-selection: After further narrowing of design features, representative semi-functional prototypes of the leading concepts were handed to users to practice the envisioned use-steps on an anatomical model. Users were asked to operate the device on the model, as if performed on themselves, to mimic the device handling, while cameras were used to record close-ups of the administration. The outcome of this HFS was a single design concept selection for detailed development.
- Usability assessment: With the chosen design concept developed into a functional prototype, users were given the opportunity to practice self-administration and simulate as many real functionalities as possible. The prototype did not contain any active drug, but did include functional features, as well as tactile and audio feedback. This level of active engagement requires the developer to conduct a risk assessment and ethics review beforehand to ensure the safety of participants.

Figure 2: Formative HFS.
It is important that HFS participants are recruited from the actual patient population. Especially with a condition as severe as cluster headaches – only those who suffer from the condition can truly represent real users (Figure 2).
Anatomical Models for In Vitro and/or Ex Vivo Tests
Determining spray deposition in the nasal cavity is challenging due to anatomical variability, variations in airflow patterns and complex fluid dynamics. Additionally, the nasal cavity presents numerous obstacles along the fluid pathway; nasal hairs, turbinate structures and mucosal surfaces can alter the spray’s trajectory and hinder its deposition in specific areas. This makes it difficult to anticipate where the spray will end up and how it will spread on the target.
Artificial 3D-models that are anatomically accurate representations of the target organ can help product design engineers visualise how the device interacts with the subject and empirically assess what interfaces can improve ergonomics, functionality and usability. This empirical approach allows for a more direct and practical assessment of spray performance, considering the actual variability seen in individuals (Figure 3).
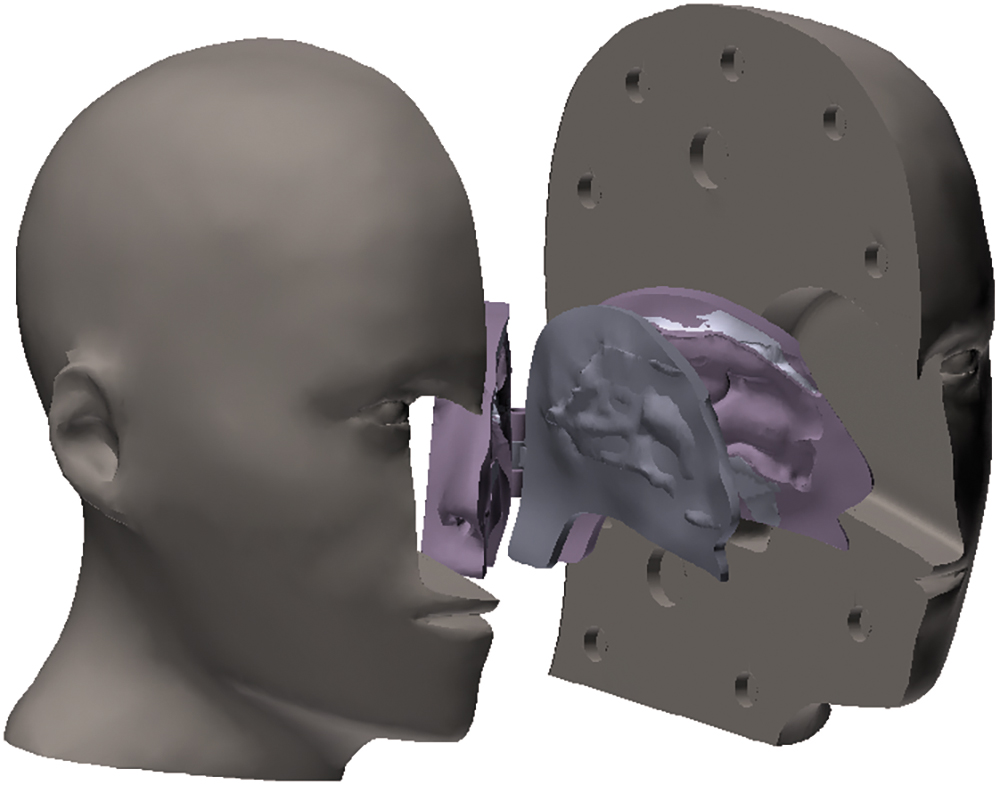
Figure 3: Nasal anatomical model reconstructed from a CT scan and 3D-printed using a mix of soft and hard materials.
“Putting treatment in the hands of patients frees their dependence on external factors, and healthcare resources can once again be allocated in new directions.”
Another test model can be based on human cadaver organs, which are more realistic than the 3D models and can be particularly useful when tissue surface plays a significant role and live animal models cannot replicate the relevant topography.
While the primary aim of these tests is to assess the device’s technical performance, they also provide valuable insights into its usability and ergonomics. For instance, the ease of device actuation, the force required for operation and the comfort of the device’s interface with the nasal anatomy can be inferred from these models. These factors are critical for ensuring that the device can be used correctly and comfortably by the intended patient population, including individuals with varying levels of manual dexterity or strength, such as the elderly or those with disabilities.
Training Materials
For patients to take control over their own treatment regime, they must be motivated to do so, as well as educated on the condition, treatment and benefits to them. Providers should not take patients’ co-operation for granted simply because compliance is within their own interest. Training aids include:
- Instructions for use (IFU): One of the key nuances in developing training materials for such products lies in striking a balance between simplicity and detail. While it is essential to provide users with clear, step-by-step instructions, it is equally important not to overwhelm them with excessive information. Human factors engineering (HFE) experts employ user-centric design principles to create training materials that prioritise the most critical information while minimising cognitive load.
- Instructional videos: Considering the diverse range of users who may interact with these products, training materials must be inclusive and accessible to individuals with varying levels of health literacy and technological proficiency. This may involve employing visual aids, plain language instructions and multimedia formats to enhance comprehension and retention. To help patients comprehend SPRACISE’s delivery method, Clexio combined the use of a live actor (more relatable) and animation of the internal organ (allowing an “inside” view).
- One-on-one guidance: Useful to evaluate the learning curve and gather unforeseen insights. While printed or digital instructions serve as valuable references, personalised guidance allows for real-time clarification of doubts and tailored assistance based on the individual’s unique needs and abilities. This personalised approach not only fosters a deeper understanding of the product and its usage, but also instils confidence in the user, reducing the likelihood of errors or misuse.
The training material, as well as its effectiveness, could be assessed during one of the HFSs. It is useful to prepare an initial IFU during the early concept design stage, based on the product’s storyboard with illustrated use steps.
CONCLUSIONS
The burden on healthcare systems never seems to reduce. As the population continues to age, healthcare services will always have their hands full. Putting treatment in the hands of patients frees their dependence on external factors, and healthcare resources can once again be allocated in new directions.
In the absence of a familiar mental model, HFE plays an ever-more critical role in the development of self-administered products, as it helps bridging the gap between the user’s expectations and the device’s capabilities. By taking into account the needs and limitations of the end-users, manufacturers can create products that are safe, effective and user-friendly. Through iterative testing and evaluation, designers can identify and address potential use errors to ensure that the product is intuitive and easy to use.
Ultimately, a well-designed self-administered drug-device combination product not only enhances patient adherence but can potentially improve clinical outcomes by ensuring correct operation. In this light, developers of truly innovative products must tailor HFE practices to the particular challenges that novice users may face.
To find out more about Clexio’s work around targeted nasal drug delivery, visit: www.clexio.com/pipeline/cle-500.
REFERENCES
- Sluder G, “The role of the sphenopalatine ganglion in nasal headaches”. NY State J Med, 1908, Vol 27, pp 8–13.
BIBLIOGRAPHY
- Towns JK, “Human factors studies for injectable combination products: from planning to reporting”. PDA J Pharm Sci Technol, 2014, Vol 68(4), pp 347–361.
- Bailey R, “Foundational human factors engineering concepts for the design of combination products”. Med Device Online, Feb 2024.
- Strochlic A, Limpens Y, Larson K, “An introduction to FDA’s Human Factors Engineering Requirements for Drug-Device Combination Products”. Pharma Focus Asia, 2023
- “Guidance on applying human factors to medical devices”. UK Medicines and Healthcare products Regulatory Agency, Sep 2019.

