To Issue 161
Citation: Chaudhuri B, Ceyssens F, “Tackling Iron-Deficiency Anaemia: a Leap Forward with a Revolutionary Buccal Patch”. ONdrugDelivery, Issue 161 (May/Jun 2024), pp 28–31.
Buddhadev P Chaudhuri and Frederik Ceyssens discuss the challenges of iron-deficiency anaemia and introduce Keylika’s new buccal patch for iron delivery.
There is currently a silent epidemic on a massive scale among us, with hardly anybody talking about it: iron-deficiency anaemia (IDA), by far the most prevalent nutritional disorder in the world. According to the WHO, the estimated prevalence of iron deficiency (ID) is 30% of the world’s population.1 Roughly 15%–25% of this can be categorised as moderate-to-severe ID. This condition, characterised by an insufficiency of red blood cells or haemoglobin in the blood, results in a diminished capacity to carry oxygen throughout the body, impairing both physical and cognitive performance. Individual sufferers complain of lack of energy, weakness and significant cognitive impairment, with devastating consequences in countries with limited healthcare capacity. IDA is more prevalent among women of childbearing age, with approximately half a billion sufferers worldwide.2
High doses of iron supplements have historically been the primary treatment for IDA, but this therapy has many disadvantages, for example, iron is incredibly inefficiently absorbed and not well tolerated most of the time, with chronic gastrointestinal (GI) side effects that few people manage to avoid. Iron supplementation became proverbial as a therapy in which the cure was often deemed worse than the disease. Intravenous (IV) iron infusion typically works better than supplements. However, it can still cause life-threatening hypersensitivity reactions and introduces its own set of complications – it is invasive and necessitates clinical administration, thus significantly increasing healthcare costs.
“Innovation is imperative to combat the IDA epidemic effectively.”
Innovation is imperative to combat the IDA epidemic effectively. Therefore, biotechnology startup Keylika is developing the world’s first resorbable buccal patch for iron delivery. This wearable patch, along with the drug design itself, has the potential to redefine the paradigm of anaemia management and IDA drug delivery.
ANAEMIA
There are multiple reasons why IDA can develop, including nutritional deficiencies; chronic blood loss from medical conditions, such as GI ulcers or heavy menstrual bleeding; a need for extra iron from pregnancy or infancy; loss of iron from the gut or malabsorption of oral iron due to underlying medical conditions, such as chronic kidney disease or inflammatory bowel disorders; resection of parts of the GI system; or long-term use of drugs that block iron absorption.
IDA causes a reduction in haemoglobin, which reduces the capacity of the blood to transport oxygen. This impacts individuals across multiple dimensions, compromising their quality of life. In its early stages, the condition can result in subtle but noticeable symptoms, including fatigue, weakness and pallor, and can be accompanied by more dramatic symptoms, such as shortness of breath, dizziness and unusual food cravings.
Severe forms of the disease can significantly limit one’s capacity to accomplish daily activities, reduce productivity at work and school, inhibit physical development in children and, eventually, lead to neurocognitive impairment. In addition to the effects on wellbeing, IDA can amplify the adverse effects of pre-existing illnesses, further compounding the cumulative health burden.
Prompt diagnosis and treatment of the condition are critical to lessen the negative impact on the lives of those afflicted. Sadly, it is severely underdiagnosed, and its symptoms are often dismissed as merely psychological in cause.
THE CURRENT STANDARD OF CARE
The current treatment options for IDA focus on replenishing the body’s iron stores and addressing the underlying causes of anaemia. Treatments can be administered orally or intravenously, depending on the severity of the condition and the individual’s specific health profile.
Oral Iron Supplements
Initially, clinical providers will prescribe an oral iron supplement for mild-to-moderate IDA, which is the most common treatment. These supplements are typically available in the form of ferrous sulfate, ferrous gluconate, ferrous fumarate, ferrous bis-glycinate or haem iron. The latter is a more efficient source but has been associated with colorectal cancer.3 The oral route is preferred for its convenience, cost-effectiveness and generally good patient compliance at low doses. However, higher doses (50–120 mg/day of elemental iron) are typically accompanied by a range of GI side effects, such as bloating, nausea, constipation, upset stomach or diarrhoea, sometimes necessitating adjustments in dosage or the type of iron supplement. Additionally, the absorption of oral iron can be influenced by factors such as the presence of certain types of food (e.g. phytates), specific medications and the overall health of the GI tract, necessitating mindful timing and management of supplement intake for optimal effectiveness.
IV Iron Therapy
In cases where oral iron supplements are ineffective, poorly tolerated or in situations of severe IDA, IV iron therapy may be employed. IV iron is directly infused into the bloodstream, bypassing the GI tract and eliminating many of the side effects of oral supplements. This method is particularly beneficial for individuals with conditions that impair oral iron absorption, require rapid replenishment of iron stores or have acutely severe anaemia needing quick resolution.
Intravenous iron is also indicated in certain situations, such as chronic kidney disease or in the pre-operative setting, where it has been shown to improve outcomes. While IV iron therapy is highly effective, there is a risk of serious adverse effects due to the high payload of iron administered at once. These include the rare, but potentially fatal, risk of anaphylactic shock. Therefore, the treatment requires medical supervision in a hospital setting, making it less accessible and more financially burdensome to the patient and the healthcare system. Depending on the type of insurance coverage, clinic and prescribed iron drug, out-of-pocket patient costs can range from US$1,000 to US$5,000 (£800–£4,000) per infusion. Additionally, IV iron can only be prescribed by a specialist (not the primary care physician) and may involve long wait times at the infusion clinic.
“Recent scientific advancements in the treatment of anaemia, particularly IDA, have led to the development of more sophisticated and targeted treatment options.”
INCREMENTAL ADVANCEMENTS IN IV AND ORAL IRON SUPPLEMENTS
Recent scientific advancements in the treatment of anaemia, particularly IDA, have led to the development of more sophisticated and targeted treatment options, aiming to improve efficacy, reduce side effects and enhance patient compliance.
Newer oral iron supplements, such as iron amino acid chelates and polysaccharide-iron complexes, have been designed to enhance iron absorption and reduce the GI side effects associated with traditional iron supplements. Their increased bioavailability means that lower doses of iron can be effective, potentially minimising the unpleasant side effects that deter patient compliance.
Significant developments have been made in the composition and administration of products on the frontier of IV iron treatments. Newer-generation IV iron formulations, such as ferric carboxymaltose, iron sucrose and iron isomaltoside, offer the advantage of allowing larger doses of iron to be administered in a single visit without the need for a test dose due to their lower risk of allergic reactions. This advancement significantly reduces the treatment burden on patients, improving adherence and outcomes, particularly in chronic conditions requiring ongoing ID management. Still, this method remains within the limited boundaries of the current standard of care.
Nevertheless, much work remains to optimise efficacy and minimise side effects in managing anaemia. At this point, oral supplements and IV remain the two prevailing treatment classes despite their disadvantages.
NEW TECHNOLOGY ON THE HORIZON
Keylika is challenging this status quo and developing the world’s first resorbable buccal patch (Rx) for treating IDA. This patch combines the efficacy of parenterals such as IV with the ease of orals, without the drawbacks of either, while still delivering iron systemically, bypassing the gut and the first-pass metabolism. The buccal route is promising, as the buccal mucosal membrane is four to 4,000 times more permeable than the stratum corneum of the skin,4 which is a more commonly used transdermal delivery route.
“Keylika’s iron ligand complex KYLK01, combined with a resorbable transmucosal patch, has the potential to transform the standard of care for ID.”
Keylika’s iron ligand complex KYLK01, combined with a resorbable transmucosal patch, has the potential to transform the standard of care for ID. The molecule has been optimised in size (<500 Da), solubility (water), ligand selection (antioxidant), stability across a wide pH range and oxidative state (ferric). This new chemical entity (NCE) is a mixed ligand iron complex tailored for a transdermal route of delivery.
The data from Keylika’s latest in vivo pharmacokinetic (PK) study in iron-deficient hamsters demonstrates efficacious and safe systemic iron absorption with the resorbable buccal patch. The patch adheres to the inner cheek of the mouth and dissolves fully in under an hour, releasing the iron drug.
Keylika’s technology aims to provide a highly productive, safe and economical solution for ID.
The Iron Complex
The metallodrug complex KYLK01 embodies several differentiators to previous and existing iron drugs and delivery mechanisms:
- The ligands are selected to enhance iron absorption and serve as an antioxidant specifically.
- The complex is small (<500 Da), increasing the ease of skin permeation.
- Its high solubility dramatically increases drug payload for a therapeutically relevant dosage.
- The ferric ion (Fe3+) form of iron aids in a seamless uptake by the iron protein transporter (transferrin) in blood plasma.
- The iron transport occurs by passive diffusion driven by a concentration gradient with the resorption of the patch in an oral salivary environment, without the need for additional complex technology aids (such as microneedles, iontophoresis, etc) to permeabilise the skin.
- The new drug complex does not contain a large-molecule carbohydrate moiety, minimising the chances of hypersensitivity reactions. Instead, it is composed of generally recognised as safe natural compounds that follow well-known metabolic pathways.
The result is an effective, safe and highly bioavailable iron form.
Patch Formulation
Keylika’s patch (Figure 1) formulation is a proprietary microemulsion-based solution that combines water-soluble polymers (USP-grade), terpenes-based permeation enhancers, dissolution rate-determining components and the water-soluble iron compound KYLK01. This formulation was first synthesised in a solution form, which was used for the In Vitro Permeation Test in a Franz Diffusion Cell (IVPT FDC) study on porcine buccal skin. The solution was then poured into moulds and baked under a vacuum to produce resorbable patches encapsulated with iron. KYLK01’s high water solubility allows for a maximum elemental iron payload of around 50 mg in the patches.

Figure 1: The buccal patch with iron ligand complex KYLK01.
In vitro dissolution studies in diluted saliva have demonstrated the full release of the iron payload (tested patches containing 30 mg Fe) in under an hour, as confirmed by inductively coupled plasma atomic emission spectroscopy.
In Vivo PK Study
ID was induced in hamsters by feeding them an iron-deficient diet over an 8-week period. Baseline serum iron levels were measured, followed by the application of a resorbable patch in the buccal pouch of the animals. Hamsters were divided into two groups of four animals each: commercial ferric citrate (control) and test (KYLK01). Animals were under light isoflurane anaesthesia during this study. The control group was administered a commercially available iron supplement, ferric citrate, while the test group received KYLK01, both in resorbable patches. Blood was sampled at intervals of 30 minutes and analysed for serum iron. Serum iron levels increased significantly over time in the test group. In contrast, the control group showed almost no iron uptake, indicating that KYLK01 has a much better systemic absorption profile than iron (III) citrate (Figure 2). Each patch was loaded with 6 mg elemental Fe.
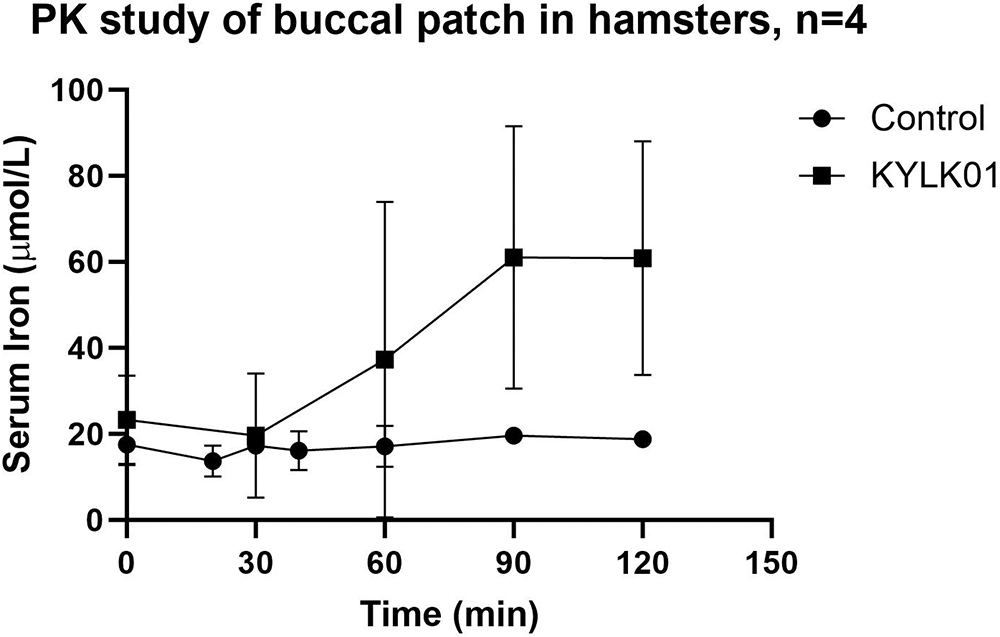
Figure 2: PK study of buccal patch in hamsters.
“The oral cavity minus the buccal pouch was also evident, demonstrating that the buccal patch remained in place, dissolved in the cheek pouch and was absorbed systemically via the transmucosal route only.”
To investigate the chances of oral ingestion of the buccal patch, a necropsy was performed with a pathological examination of the entire GI tract. No evidence of the coloured patch or remnants was found throughout the whole tract, including the oesophagus, stomach and intestines. The oral cavity minus the buccal pouch was also evident, demonstrating that the buccal patch remained in place, dissolved in the cheek pouch and was absorbed systemically via the transmucosal route only. No signs of buccal irritation or systemic toxicity were observed.
The significantly superior PK profile with KYLK01 buccal patches is preliminary proof that:
- KYLK01 is systemically absorbed via the transmucosal route
- The resorbable patch formulation successfully dissolves in saliva, releasing the iron for buccal uptake
- KYLK01’s rational molecular design, tailored for a buccal delivery, is successful, as opposed to using iron (III) citrate.
CONCLUSION
ID is a hugely underserved problem supported by a sub-par standard of care. The limitations of current treatments and the transformative potential of this innovation make it evident that Keylika is not merely introducing a new treatment option but instead changing the paradigm of IDA treatment with the first-ever buccal delivery of iron. The transport of this novel iron complex through the highly vascularised oral mucosa into systemic circulation circumvents the GI tract and associated adverse side effects. As a convenient point-of-care solution, the patch could potentially eliminate the compliance-limiting side effects of oral supplements while erasing the cost, stress, risk and time requirement of IV iron infusions, heralding a new era of convenience, efficacy and safety in ID treatment. The success of early animal studies boosts Keylika’s confidence that it can also employ the buccal route for a multitude of hard-to-deliver drugs, from small to large molecules.
REFERENCES
- Kumar A et al, “Iron deficiency anemia: pathophysiology, assessment, practical management”. BMJ Open Gastroenterol, 2022, Vol 9(1), Article e000759.
- “Anaemia”. WHO, May 1, 2023.
- Aglago EK et al, “Dietary intake of total, heme and non-heme iron and the risk of colorectal cancer in a European prospective cohort study”. Br J Cancer, 2023, Vol 128, pp 1529–1540.
- Wanasathop A et al, “Permeability of Buccal Mucosa”. Pharmaceutics, 2021, Vol 13(11), p 1814.

