To Issue 161
Citation: Hunt J, Zhang Y, “Adapting to an Ageing World: Formulation Strategies to Boost Patient Compliance”, ONdrugDelivery, Issue 161 (May/Jun 2024), pp 22–26.
Jason Hunt and Yeli Zhang discuss the key factors undermining patient compliance in older adults and explore how the pharmaceutical industry can leverage excipients to enhance drug formulations and improve medication compliance among this critical demographic.
The ageing population is an undeniable global trend, with a profound influence on the world’s demographic landscape. The number of people aged 65 years or older worldwide is projected to more than double by 2050, rising from 761 million in 2021 to 1.6 billion.1 This naturally raises several challenges in healthcare; as we age, the risk of developing chronic diseases increases, meaning the number of people with healthcare needs is on the rise. In the US alone, an evidence-based model predicts that the number of adults aged 50 years and older with at least one chronic disease will double from 71.5 million in 2020 to 142.7 million by 2050, underscoring the difficulties posed by the ageing trajectory.2
This rapid population ageing and escalating chronic disease burden are putting unprecedented pressure on the pharmaceutical industry to address the medical needs of the expanding senior demographic more effectively. However, to make any meaningful impact, drug manufacturers must tackle a pivotal issue for this age group – poor patient compliance.
IMPLICATIONS OF AN AGEING POPULATION ON THE PHARMACEUTICAL INDUSTRY
The healthcare challenges presented by the world’s ageing population have significant implications for the global economy and healthcare systems. In the UK, for instance, individuals over the age of 65 account for more than 40% of hospital admissions and occupy around 60% of inpatient beds, as well as being the most frequent users of health and social care services.3 Alarmingly, the cost associated with the rising prevalence of ageing and chronic diseases is estimated to reach a staggering US$47 trillion (£37 trillion) globally by 2030.4
For the pharmaceutical industry, this translates to a surge in demand for certain medications. This trend will primarily impact drugs that address the most prevalent chronic conditions affecting senior individuals. These include:5
- Cardiovascular disease
- Hypertension
- High cholesterol
- Arthritis
- Coronary heart disease
- Diabetes
- Chronic kidney disease
- Heart failure
- Depression
- Alzheimer’s disease
- Dementia
- Chronic obstructive pulmonary disease.
The increased prevalence of comorbidities in ageing populations – when individuals are affected by multiple conditions simultaneously – adds further pressure to the need for age-related disease medications. Notably, the prevalence of comorbidities in US adults aged 50 and older is projected to rise from 7.8 million in 2020 to 15 million by 2050.2
Beyond providing the necessary medications to seniors, healthcare providers also face the major issue of poor compliance among this demographic. But why is this the case – and how can it be addressed?
TACKLING THE PERVASIVE CHALLENGE OF POOR PATIENT COMPLIANCE IN SENIORS
Several factors contribute to poor patient compliance among older patients. For example, those suffering from comorbidities are more likely to have complicated treatment regimens that are more difficult to follow. The ageing process can also impact cognition and memory, leading to forgetfulness – meaning senior patients are at an increased risk of missing or incorrectly taking their medications.
Additionally, some older adults have difficulty swallowing oral medicines, a condition known as dysphagia, which can significantly compromise compliance. Many of these individuals resort to crushing tablets or opening capsules to overcome this issue, which can alter drug absorption and lead to serious consequences, such as under- or overdosing, or even fatality.6
“The implications of poor medication adherence are profound, contributing to as much as 50% of treatment failures in the US, as well as 125,000 deaths and up to 25% of hospitalisations every year.”
The implications of poor medication adherence are profound, contributing to as much as 50% of treatment failures in the US, as well as 125,000 deaths and up to 25% of hospitalisations every year.7 Addressing this is therefore crucial for improving health outcomes and quality of life among the ageing population. So, what formulation strategies can drug developers implement to make their medications more suitable and appealing to senior adults?
Optimise Dosing With Controlled-Release Formulations
The pharmaceutical industry experienced a pivotal breakthrough over 50 years ago with the development of oral and transdermal controlled-release (CR) drug formulations.8 These innovative delivery systems allow for the gradual release of an API in the body, revolutionising medication administration and offering several advantages over conventional oral dosage forms. Among the many advantages of CR formulations is improved patient compliance.9
It is not just patients that benefit from CR systems, but formulators too. CR formulations enable drug manufacturers to modulate the pharmacokinetic profile of the API, leading to more predictable, consistent and sustained drug levels in the bloodstream.9 This means that patients may not need to take as much medicine to reap the benefits, which has the potential to reduce pill burden, further improving compliance. Moreover, these specialised delivery formats can help to minimise drug level fluctuations, mitigating the risk of adverse reactions.9
In addition, some CR technologies can improve bioavailability. For example, certain cyclodextrins complexed with poorly water-soluble APIs can improve the solubility, and possibly the permeability, of the API, thereby enhancing the bioavailability while also providing controlled release.10 Prodrugs are another method that can potentially improve bioavailability and, if so designed, can support controlled release.11 CR matrices and barrier coatings may also provide added benefits in protecting the API within the formulation from enzymatic or pH degradation. However, these technologies do not protect APIs that have already been released from the formulation.
CR formulations can also address the unique needs of different patient populations. Drug manufacturers can customise CR delivery systems to achieve specific release profiles tailored to individual therapeutic outcomes. For example, CR transdermal patches have recently been developed for administering carvedilol, a beta-blocker drug used to treat hypertension and heart failure – a common disease among the elderly.12 The matrix design of these innovative patches enables the slow, sustained release of the drug through the skin, reducing the frequency of administration for patients who have difficulty swallowing, are more forgetful or who already take several other pills.12
Overcome Swallowing Challenges With ODTs
Solid oral dosage forms are the default formulation for most drugs, due to their convenient administration, accurate dosing, manufacturing efficiency and widespread acceptance. However, as mentioned prior, pills and capsules can be difficult or unappealing to swallow for some senior patients.13 To address this, innovators can consider strategies, such as smaller tablets or capsules, easy-to-swallow film coatings or novel oral delivery systems, including liquid formats or orally disintegrating tablets (ODTs).
Designed to rapidly disintegrate in the mouth without the need for water, ODTs enable easy administration for patients who struggle with conventional solid dosage forms.14 One way to achieve this simple method of administration is through the incorporation of superdisintegrants, which facilitate rapid tablet breakdown upon contact with saliva. In some cases, the intent is for the API to be absorbed through the oral mucosa, bypassing first-pass metabolism in the gastrointestinal tract. ODTs can facilitate this, potentially offering superior bioavailability compared with traditional tablet and capsule formulations.14
The benefits of this patient-centric delivery method have been demonstrated in several clinical studies, including one investigating the efficacy and tolerability of mirtazapine ODTs in depressed elderly nursing home residents with complex medical and cognitive comorbidities.15 The results of this specific trial revealed that the rapidly dissolving tablets provided effective antidepressant action and were also well-tolerated in these “difficult-to treat” patients.
“By overcoming swallowing difficulties through innovative formulation strategies, ODTs present a simple yet impactful solution
for improving medication adherence among the elderly.”
By overcoming swallowing difficulties through innovative formulation strategies, ODTs present a simple yet impactful solution for improving medication adherence among the elderly. However, not all APIs are well-suited to this format, which is why palatability is a focus for some manufacturers.
Enhance Palatability to Boost Adherence
While palatability might seem like an obvious influencer of patient compliance, it is often overlooked in the pharmaceutical industry.16 Implementing strategic taste-masking techniques to conceal the bitter or unpleasant taste of drugs can significantly improve medication acceptance for many patients. Taste-masking approaches typically fall into two major categories, as shown in Figure 1. The first involves modifying the perception of unpleasant taste through the addition of more palatable ingredients, such as flavouring agents, sweeteners or bitterness inhibitors.17 This method is suitable for APIs with low-to-moderate bitterness but can prove challenging for highly bitter drugs.
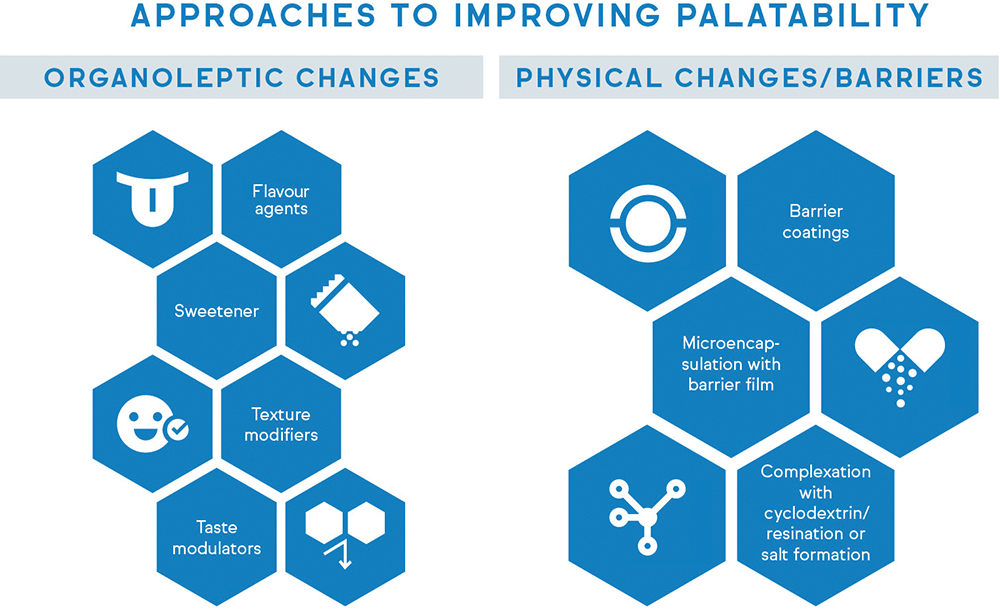
Figure 1: Taste-masking approaches typically fall into two major categories – organoleptic changes and physical alterations.
“Through taste-masking strategies, pharmaceutical manufacturers can develop drug products that are not only efficacious, but also more readily accepted by senior patients – a pivotal consideration for long-term medication compliance.”
The alternative strategy is to physically or chemically alter the properties of the unpalatable API to prevent direct contact with the patient’s taste receptors.17 This can be achieved through complexation, encapsulation or other techniques that create a barrier between the bitter compound and the taste buds. For example, ethyl cellulose can be used as a barrier membrane to prevent the tongue from experiencing the taste of bitter APIs, such as rupatadine fumarate and paracetamol.18,19 Through taste-masking strategies, pharmaceutical manufacturers can develop drug products that are not only efficacious, but also more readily accepted by senior patients – a pivotal consideration for long-term medication compliance.
THE CRUCIAL ROLE OF EXCIPIENTS
All the solutions and strategies discussed in this article would not be possible without excipients. While excipients may not possess inherent therapeutic effects, they play an essential role in optimising drug formulations. The following are some examples of how these versatile pharmaceutical ingredients can make a meaningful impact on patient drug compliance.
Facilitating CR Delivery
Functional excipients are crucial to the success of CR formulations, as their unique properties help modulate the release kinetics of APIs. Factors such as excipient chemistry, particle size and molecular weight can all influence CR performance.20,21 For example, many innovative CR products leverage high-molecular weight, water-soluble cellulose ethers such as hypromellose (HPMC) to attain CR performance. Further fine-tuning can be achieved by adjusting excipient substitution levels – for HPMC, varying the degree of hydroxypropyl substitution may impact hydrophilicity and, in turn, CR performance.
Enabling ODT Formulation
Excipients are necessary components of ODTs, each serving a distinct purpose in the formulation. For example, croscarmellose acts as a superdisintegrant, promoting rapid disintegration of ODTs upon contact with saliva.22 On the other hand, excipients such as mannitol provide excellent compactability and act as a bulking material to produce ODTs with robust physical properties.22 Excipients can also be incorporated into ODTs to provide specific functions, such as flowability, compressibility, palatability and dissolution enhancement.
Masking Unpleasant Tastes
Excipients can be leveraged as physical barriers or solubility modifiers to improve the palatability of bitter or unpleasant tasting drug products.17 For example, polymer coating technologies can create a protective layer that prevents direct API-taste receptor interactions. Depending on the drug’s bitterness profile and formulation properties, APIs can be coated with excipient polymers that have either a pH-dependent (e.g. hypromellose phthalate) or pH-independent (e.g. ethylcellulose) release profile. The coating level on the API or dosage form can be optimised to successfully achieve taste masking.
CONCLUSION
It is evident that addressing patient compliance among the ever-growing ageing population is an urgent need for global healthcare systems. By leveraging advanced drug delivery technologies and specialised excipients, pharmaceutical companies can develop more patient-centric formulations to improve outcomes for older patients. From CR systems and dissolvable tablets to effective taste-masking alternatives, these innovations can increase the odds that patients will take their medications as prescribed and improve their quality of life. This will, ultimately, have a ripple effect beyond individual patients, offering broader benefits for the global economy and healthcare systems.
REFERENCES
- “International Day of Older Persons 1 October”. Web Page, United Nations, accessed May 2024.
- Ansah JP, Chiu C-T, “Projecting the chronic disease burden among the adult population in the United States using a multi-state population model”. Front Public Health, 2022, Vol 10, Article 1082183.
- “Protecting the rights of older people to health and social care”. British Geriatrics Society, Jan 2023.
- “Methodological Appendix: The Global Economic Burden of Non-Communicable Diseases”. World Economic Forum & Harvard School of Public Health, Sep 2011.
- “The Top 10 Most Common Chronic Conditions in Older Adults”. National Council on Aging, Apr 2024.
- “Crushing tablets or opening capsules: many uncertainties, some established dangers”. Prescrire Int, 2014, Vol 23(152), pp 209–211, 213–214.
- Kim J et al, “Medication Adherence: The Elephant in the Room”. US Pharm, 2018, Vol 43(1), pp 30–34.
- Ezike TC et al, “Advances in drug delivery systems, challenges and future directions”. Heliyon, 2023, Vol 9(6), Article e17488.
- Adepu S, Ramakrishna S, “Controlled Drug Delivery Systems: Current Status and Future Directions”. Molecules, 2021, Vol 26(19), Article 5905.
- Real DA et al, “Cyclodextrin- Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability”. Pharmaceutics, 2021, Vol 13(12), Article 2131.
- Zhang X et al, “Facile Fabrication of 10-Hydroxycamptothecin-Backboned Amphiphilic Polyprodrug with Precisely Tailored Drug Loading Content for Controlled Release”. Bioconjug Chem, 2018, Vol 29(7), pp 2239–2247.
- Mo L et al, “Formulation and development of novel control release transdermal patches of carvedilol to improve bioavailability for the treatment of heart failure”. Saudi J Biol Sci, 2022, Vol 29(1), pp 266–272.
- Forough AS et al, “A spoonful of sugar helps the medicine go down? A review of strategies for making pills easier to swallow”. Patient Prefer Adherence, 2018, Vol 12, pp 1337–1346.
- Hannan PA et al, “Oral Dispersible System: A New Approach in Drug Delivery System”. Indian J Pharm Sci, 2016, Vol 78(1), pp 2–7.
- Roose SP et al, “Open-label study of mirtazapine orally disintegrating tablets in depressed patients in the nursing home”. Curr Med Res Opin, 2003, Vol 19(8), pp 737–746.
- “The importance of taste in pharmaceutical development”. Manufacturing Chemist, Oct 2017.
- “Taste Masking 101: Optimize Taste And Improve Patient Outcomes”. Webinar, Adare Pharma Solutions, Jun 2021.
- Wasilewska K et al, “Ethylcellulose in Organic Solution or Aqueous Dispersion Form in Designing Taste-Masked Microparticles by the Spray Drying Technique with a Model Bitter Drug: Rupatadine Fumarate”. Polymers (Basel), 2019, Vol 11(3), Article 522.
- Alhamidi R, Ibrahim W, “Preparation and Evaluation of Taste Masked Paracetamol Microcapsules”. Res J Pharm Technol, 2022, Vol 15(8), pp 3703–3708.
- Heng PW et al, “Investigation of the influence of mean HPMC particle size and number of polymer particles on the release of aspirin from swellable hydrophilic matrix tablets”. J Control Release,
2001, Vol 76(1–2), pp 39–49. - Dahl TC et al, “Influence of physico-chemical properties of hydroxypropyl methylcellulose on naproxen release from sustained release matrix tablets”. J Control Release, 1990, Vol 14(1), pp 1–10.
- Ghourichay MP et al, “Formulation and Quality Control of Orally Disintegrating Tablets (ODTs): Recent Advances and Perspectives”. Biomed Res Int, 2021, Vol 2021, Article 6618934.

