To Issue 162
Citation: Fuchs C, Ram R, “Optimising Inhalation Adherence and Technique: The Power of Digital Technology”. ONdrugDelivery, Issue 162 (Jun 2024), pp 16–20.
Carola Fuchs and Rupa Ram discuss the development of PARI’s new eFlow Integrated Nebuliser and the PARI Breath Guide app, and how integrating these into the PARI Connect Platform will stimulate increased adherence to inhaled therapy and, therefore, improved therapeutic outcomes.
REGULAR, CORRECT INHALATION IS THE FOUNDATION FOR OPTIMAL CLINICAL OUTCOMES
Increasing patient adherence to long-term treatment is a beneficial and powerful factor for optimising the effectiveness of therapy in chronic diseases. Better adherence has been shown to reduce disease exacerbations and the need for emergency therapy or further medical intervention, as well as improve clinical outcomes.1,2 However, around half of patients with chronic illness fail to take their medications as prescribed,3,4 increasing the risk of their disease worsening.5
Patients with chronic respiratory diseases often rely on inhaled therapies. For these patients, successful treatment not only requires efficacious drugs, but also efficient delivery of the drug to the lungs and full patient adherence. Drug delivery to the lungs can be optimised by using a high-performance nebuliser combined with good inhalation technique.6 Furthermore, adherence can be improved if patient acceptance of the device is high.7 So how can both be ensured?
In recent years, the use of digital tools has rapidly gathered momentum across all areas of medicine.8 The scope and potential of these tools is vast, offering personalised support and data-driven decision making, increasing patient satisfaction and adherence and, ultimately, improving therapy outcomes.9 For patients with chronic respiratory diseases, digital tools can provide transformative benefits that maximise therapeutic success by assisting patients in using their nebuliser correctly, such as by providing feedback on each treatment and sharing real-life clinical data with healthcare professionals.
“Patient monitoring and increasing adherence have been a focus for
PARI during the development of the PARI Connect Platform.”
Patient monitoring and increasing adherence have been a focus for PARI during the development of the PARI Connect Platform. The platform has now been expanded to include unique features that aim to improve patients’ inhalation technique and promote device acceptance. The platform includes the PARI Breath-Guide app, specially developed for the new eFlow® Integrated Nebuliser and designed to improve both usability and therapy support.
PARI: ENHANCING CONNECTIVITY WITH ALMOST 10 YEARS’ EXPERIENCE
Since 2015, PARI has offered monitoring features for its customised vibrating membrane nebulisers for patients with chronic respiratory diseases. The eTrack® Controller is a Bluetooth- and WiFi-ready controller for the eFlow® technology nebuliser platform that, in addition to operating the nebulisation, allows automatic transfer of inhalation data to secure cloud storage without the need for the patient to actively transfer the data after each treatment.
Within the PARI Connect Platform, patients can use one of PARI’s apps to transfer their nebuliser data to the PARI Cloud for subsequent analysis and visualisation. These apps are coupled with the PARItrack® Dashboard, which aggregates and displays the data for physicians, caregivers and/or study personnel (Figure 1). Together, these components offer transparent data for both patients and physicians on the use of and adherence to nebuliser therapy, giving them the tools to intervene, adapt and optimise therapy outcomes.
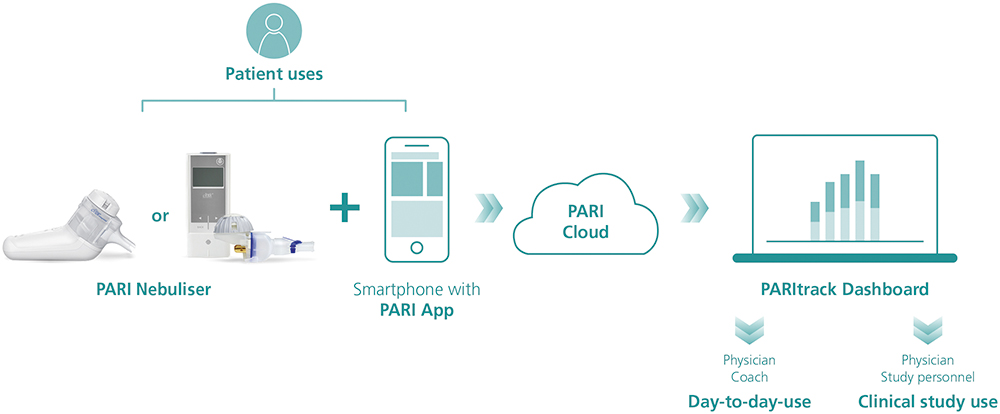
Figure 1: The PARI Connect platform.
The PARI Connect® app and PARItrack® Dashboard
The PARI Connect® app has been available since 2021 and offers several features to support patients with their therapy management and to document adherence. These include personally adjustable therapy reminders, graphical visualisation of therapy data to see long-term trends, automatic messages when adherence decreases and support from family and friends through a “buddy system”.
The PARItrack® Dashboard is a web portal that provides physicians or study personnel with easy access to aggregated patient data in graphical and tabular form. The system’s focus is on therapy adherence, but also includes patients’ vital parameters, creating a strong basis for patient support both in day-to-day use and in clinical studies.
PARI Connect: Improvements in Adherence
The potential of the PARI Connect Platform has been demonstrated, with improved patient adherence shown in both clinical trials of new drug products and real-world settings.
“Over the last nine years, around 5,000 patients have used the eTrack® Controller together with their eFlow® Technology nebulisers in clinical trial settings.”
Over the last nine years, around 5,000 patients have used the eTrack® Controller together with their eFlow® Technology nebulisers in clinical trial settings, alongside 13 partners across Europe, the UK, the US, Israel, Australia and Canada. Within these trials, data were shared according to the study protocol, enabling physicians to contact patients with poor adherence to offer support and ensure correct and regular nebuliser use. Mean adherence rates were between 76% and 99% for studies with durations of up to two years and four to eight weeks, respectively.10 The high adherence rates seen in these studies not only provide the best opportunity for maximising treatment efficacy, but also allow accurate evaluation of study outcomes and may lead to savings in cost and time due to a reduction in the number of patients needed to be recruited.11
Additionally, studies in the UK and Germany investigating the impact of adherence support on day-to-day inhaler use have shown substantial increases in adherence where patients and physicians have access to data and analysis facilitating appropriate self-management and support.12–14 In the study sponsored by the German Innovation Fund investigating telemonitoring and coaching, the PARI Connect® app was used by patients with cystic fibrosis, together with other interventions. The interim results showed that therapy adherence was significantly higher in the patient group using the app than the control group without the app. This adherence increase was stable throughout the evaluated duration of 12 months. Notably, adherence has now been established as a key quality indicator in cystic fibrosis care by the UK NHS.
In recent years, practical experience with the PARI Connect Platform in real-world settings has grown, showing that the improvements in adherence seen in clinical trials also translate to real-world settings.10 The new eFlow Integrated Nebuliser is now becoming part of the PARI Connect Platform, compatible with the PARI Cloud and PARItrack Dashboard, leveraging the company’s experience in adherence support and offering a new dimension of therapy support with additional real-time breath-guiding during each inhalation.
BREATH-GUIDING: SUPPORTING NEBULISER THERAPIES AND OPTIMISING NEBULISATION RESULTS
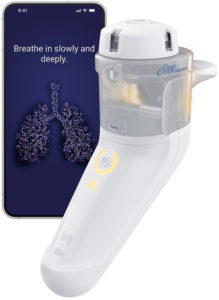
Figure 2: PARI’s eFlow Integrated Nebuliser and the PARI Breath Guide app.
The role of digital tools in supporting the correct breathing pattern is pivotal for optimising both the drug delivery to the lungs and patients’ acceptance of the device. A long inhalation phase is ideal, as it allows low peak inspiratory flow, reducing aerosol droplet impaction in the upper airways and promoting a deep breath to increase drug deposition in the lungs.
PARI’s experience and research over the last decade has shown that feedback on patients’ inhalation technique is important, both for reassurance and for guidance on required improvements. Taking this into consideration, PARI has incorporated an innovative breath-guiding feature into the eFlow® Integrated Nebuliser, which guides the patient to inhale slowly and deeply (Figure 2).
The breath-guiding concept comprises three components that work together to optimise drug deposition, reduce variability and reduce treatment time, ultimately promoting high patient satisfaction and adherence. The first of these is a unique built-in flow resistance called a “fluidic diode”. Through the specific geometry of the fluidic diode, the flow resistance is higher for inhalation than for exhalation. This limits the peak inspiratory flow, thereby increasing inspiration duration, reducing aerosol losses in the throat and increasing the residence time of aerosol droplets in the lungs to enhance sedimentation, all while also keeping treatment time to a minimum. With its reduced resistance during exhalation, this approach also enables exhalation through the nebuliser for greater patient comfort, steadiness and acceptance.
The second component is the nebuliser’s user interface, which directly provides intuitive instructions via an illumination in the patient-facing upper part of the nebuliser and a vibration of the lower part, which is where the patient holds the device during inhalation. These two feedback mechanisms effectively train patients to breathe correctly during inhalation. This is achieved using a pressure sensor that collects real-time data from the inspiratory flow and translates it into haptic and visual signals. These inform the user whenever the inhaled flow rate is too high, guiding them to inhale more slowly.
Finally, further feedback is offered simultaneously via the new connected PARI Breath Guide app, which provides a helpful real-time visualisation of the inhalation, guiding the patient to inhale both slowly and deeply, as well as informing them whether the therapy has been performed correctly and, if necessary, how to improve. This breath guiding function is provided in addition to the established adherence visualisation.
The PARI Breath Guide App Supports Breath-Guiding at Three Timepoints
The new PARI Breath Guide app offers guidance to the patient before, during and after treatment.
Onboarding Tutorial
Before treatment, the patient is provided with a step-by-step explanation of how to use the eFlow® Integrated Nebuliser for optimal results (Figures 3). Since the eFlow Integrated Nebuliser is used as a drug-device combination product, there is potential to integrate further pharma-partner-specific content here in the future.
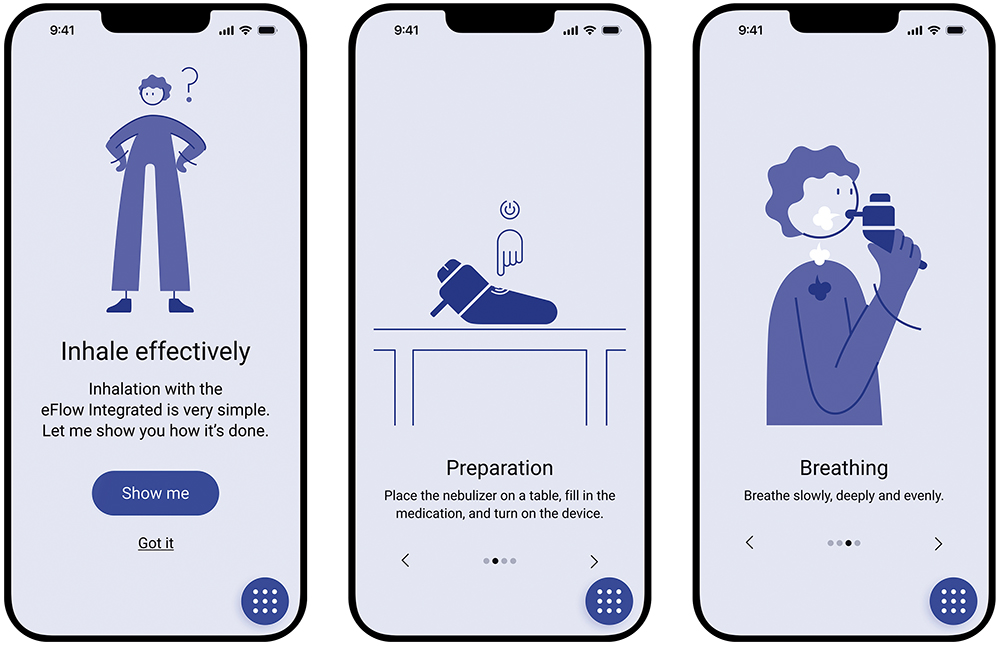
Figure 3: The PARI Breath Guide app’s onboarding tutorial.
During Inhalation
During inhalation, the app uses real-time inhalation data to provide visual guidance (Figure 4). The visualisation shows how the lung fills with aerosol based on the individually measured inhaled volume. This feature aims to motivate patients to inhale more deeply and for longer within their own capacity and has been shown to be very popular with patients in human factors studies.
The app also informs the patient immediately if they are inhaling too quickly with a significant colour change of the whole display, corresponding to the illumination of the nebuliser device itself. While the peak inspiratory flow threshold is set in the device and the app, the guidance on inhaled volume in the app dynamically adapts according to the lung capacity of the individual patient. As such, patients with the ability to achieve a high inhaled volume are motivated to breathe longer and more deeply, whereas severely ill patients who may not be able to reach such a volume are not led to become frustrated or exhausted. The dynamic adaptation also accounts for different lung sizes and daily changes in lung health and breathing capacity.
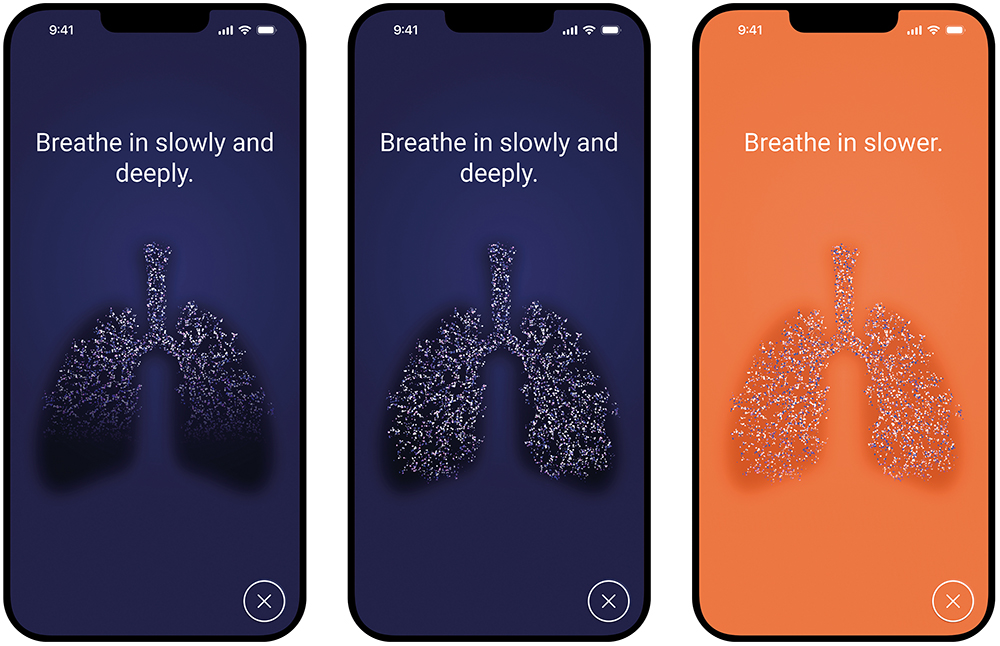
Figure 4: A sample visualisation of breath-guiding in the PARI Breath Guide app.
Post-Inhalation
At the end of each administration, the patient receives feedback on the inhalation via a detailed inhalation report to help them improve their breathing technique, where needed. The report considers the peak inspiratory flow, inhaled volume, uniformity and completion of the inhalation. The report also includes tips on what to improve and allows the patient to study their breathing pattern.
Completion of the inhalation is considered the first priority, followed by the peak inspiratory flow and inhaled volume. If these elements were performed successfully, the report will also encourage the patient to inhale more evenly. The inhalation report is created within the nebuliser device, independent of the use of the app during inhalation. These reports can help patients to continually improve and inform their physicians via the PARItrack Dashboard on their success or need for further training.
“Adding the eFlow Integrated Nebuliser with the PARI Breath Guide app to the platform will be invaluable in terms of improving patient engagement.”

Figure 5: A sample inhalation report from the PARI Breath Guide app.
THE BENEFITS OF THE PARI CONNECT PLATFORM: PATIENTS WILL HAVE THE FINAL SAY
Over 2,300 patients are currently connected to the PARI Connect Platform worldwide. Adding the eFlow Integrated Nebuliser with the PARI Breath Guide app to the platform will be invaluable in terms of improving patient engagement (Figure 5). The adherence data speak for themselves, and usability tests have shown that the user experience is enhanced by the combination of the eFlow Integrated Nebuliser, with its real-time breath-guiding features, and the PARI Breath Guide app. Furthermore, preliminary data from initial usability tests with patients show that the breath-guiding features have a positive influence on patients’ inhalation technique, with the PARI Breath Guide app additionally enhancing patient satisfaction and reassurance.
With more than 20 years of experience with its eFlow Technology platform and almost 10 years in patient-centric development and expansion of the PARI Connect Platform with proven adherence support, PARI believes that the new eFlow Integrated Nebuliser with breath-guiding functionality integrated into the PARI Connect Platform can, ultimately, improve clinical outcomes via correct, regular and efficient drug delivery for patients with chronic respiratory diseases.
ACKNOWLEDGEMENT
Martin Guppy, PhD, Medical Writer, participated in the writing and proofreading of the manuscript.
REFERENCES
- Lomas P, “Enhancing adherence to inhaled therapies in cystic fibrosis”. Ther Adv Respir Dis, 2014, Vol 8(2), pp 39–47.
- Daniels T et al, “613 Exploring FEV1 response by co-adherence to inhaled therapies in people with cystic fibrosis taking elexacaftor-tezacaftor-ivacaftor: year 1 of the NEEMO-CFHealthHub learning health system evaluation”. J Cyst Fibros, 2023, Vol 22, pp S326–S327.
- Lee JK, Grace KA, Taylor AJ, “Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial”. JAMA, 2006, Vol 296(21), pp 2563–2571.
- “Adherence to long-term therapies: evidence for action”. Report, WHO, 2003.
- Osterberg L, Blaschke T, “Adherence to medication”. N Engl J Med, 2005, Vol 353(5), pp 487–497.
- Rogliani P et al, “Optimizing drug delivery in COPD: The role of inhaler devices”. Respir Med, 2017, Vol 124, pp 6–14.
- Lavorini F, Fontana GA, “Inhaler technique and patient’s preference for dry powder inhaler devices”. Expert Opin Drug Deliv, 2014, Vol 11(1), pp 1–3.
- “Global strategy on digital health 2020-2025”. Report, WHO, Aug 2021.
- Sleurs K, “Mobile health tools for the management of chronic respiratory diseases”. Allergy, 2019, Vol 74(7), pp 1292–1306.
- Koehler Y, Fuchs C, “Monitoring Nebuliser Usage & Lung Function in Clinical Trials”. ONdrugDelivery, Issue 87 (Jun 2018), pp 66–70.
- Fiebig D, “Late Breaking Abstract – Making therapy behaviour transparent by using an app and a Bluetooth® enabled electronic nebulizer, was found to increase therapy adherence”. Oral Presentation, The ERS International Congress, Milan, 2023.
- Thee S, “A multi-centre, randomized, controlled trial on coaching and telemonitoring in patients with cystic fibrosis: conneCT CF”. BMC Pulm Med, 2021, Vol 21(1), Article 131.
- Wildman MJ et al, “An intervention to support adherence to inhaled medication in adults with cystic fibrosis: the ACtiF research programme including RCT”. Southampton (UK): NIHR Journals Library, Oct 2021.
- Wildman MJ et al, “Self-management intervention to reduce pulmonary exacerbations by supporting treatment adherence in adults with cystic fibrosis: a randomised controlled trial”. Thorax, 2022, Vol 77(5), pp 461–469.

