To Issue 163
Citation: Thompson I, “Ypsomed’s Platform Product Transformation and Approach to Strategic Partner Networking for Self-Injection Devices”. ONdrugDelivery, Issue 163 (Jul 2024), pp 24–28.
Ian Thompson describes how Ypsomed has transformed the market for platform-based self-injection devices and extended the concept of platform products beyond the core device by developing working practices with strategic networking partners, helping pharma companies and patients to source safe, reliable and easy-to-use drug-device combination products.
PHARMA – THE GREAT OUTSOURCER
In 2023, global pharma sales were estimated at US$1.6 trillion (£1.3 trillion), with the top 25 global pharma companies accounting for around 50% of these sales. Today, biologics account for more than $500 billion, or just over 30% of global pharma sales. This proportion is set to grow further as pharma develops more self-injectables in the therapy areas of oncology, neurology and rare diseases, in addition to the larger and growing markets for treating broader autoimmune diseases and insulin/glucagon-like peptide 1 (GLP-1)-based drugs for treating diabesity.
With an approximately 15% spend on R&D and average cost of goods at around 25%, pharma invests around $650 billion in R&D and operations, with as much as half outsourced to a broad range of organisations, including contract research organisations (CROs) for R&D and contract development and manufacturing organisations (CDMOs) for manufacturing. Outsourcing is a key activity for pharma companies and their supply chains have become global and complex. With more outsourcing, new modalities and novel ways to reach patients, it is critical to ensure that outsourcing networks are robust and can withstand shocks.
THE PLATFORM PRODUCT APPROACH TO SELF-INJECTION DEVICES
The platform approach for injectable drug delivery devices emerged through the use of standardised, ready-to-fill prefillable syringes, for the injection of heparins and vaccines, and prefilled cartridges, for insulin and dental lidocaine. Many of these innovations were introduced in parallel with the development and growth of the markets for these molecules in the 1980s and 1990s. During that time, the self-injection market developed with more dedicated bespoke devices as insulin and human growth hormone (hGH) were developed and needed to be dosed using an accurate pen mechanism for frequent, typically daily, injections.
In the 1990s and 2000s, the development of interferons and monoclonal antibodies (mAbs) led to the introduction of automated spring-driven autoinjectors for less frequent injections based on fixed doses. Until 2006, when the first handheld prefilled autoinjectors were launched, there were relatively few self-injection devices on the market. At that time, the market was dominated by insulin pens from major manufacturers such as Novo Nordisk, Lilly and Sanofi and the first prefilled autoinjectors for the two anti-tumour necrosis factor (TNF) antibodies Humira® (adalimumab, AbbVie) and Enbrel® (etanercept, Amgen).
“The 2000s were transformational for both injection pens and autoinjectors with the introduction of platform-based business models and a growing number of drug product launches in the 2010s.”
The 2000s were transformational for both injection pens and autoinjectors, with the introduction of platform-based business models and a growing number of drug product launches in the 2010s. The term “platform product” refers to a standardised self-injection device technology that can be adapted to the needs of a variety of drugs while being supplied from common manufacturing infrastructure. Already established in other industries, platforms serve to de-risk and accelerate the development and manufacture of a variety of customised product iterations. In contrast to bespoke devices designed for individual drugs, platform products are versatile and can accommodate varying drug requirements, such as primary packaging configuration, dose volume and drug viscosity.
There has recently been a notable shift towards customising platforms to create a spectrum of drug-device combinations, resulting in simplified access to advanced drug-device combination products for a wide range of biopharmaceutical customer segments, including small biotechnology innovators, emerging biosimilar manufacturers and global big pharma companies – the latter often adopting a platform-in-platform approach to further streamline the serial launch of injectable drug-device combination products with proven supply chains, partnerships and manufacturing infrastructures.
YPSOMED’S PLATFORM PRODUCT PARTNERSHIP STRATEGY
Pharma has developed four key elements to improve supply-chain resilience for its drug products:
- Mapping suppliers with end-to-end transparency to fully understand the supply chain and identify potential vulnerabilities
- Performing routine stress testing and reassessment, often entailing regular audits of critical suppliers
- Reduction in exposure to shocks through multisourcing and striking a balance between just-in-time and just-in-case inventory levels
- Ensuring that supply-chain risk and resilience is embedded in both strategic planning and day-to-day execution, with structured governance to ensure that decisions are made and acted on at the right level and time.
All of these aspects are mirrored within Ypsomed Delivery Systems’ structures and processes, which are designed to develop long-lasting and trusting partnerships with pharma customers. Of these points, end-to-end transparency and structured governance are especially important aspects within the strategic partner networks of suppliers that Ypsomed maintains to benefit pharma clients and patients alike.
COLLABORATIVE PARTNERSHIPS AND REGULAR AUDITS
Ypsomed supports a wide range of small biotech, mid-size pharma and big pharma customers around the globe. The complexity of the pharma supply chain demands an open exchange on technical, logistical and commercial topics. Ypsomed’s cross-industry expertise in drug delivery is highly valued by its customers and its specialists have guided numerous customers through the design and development of innovative self-injection devices, as well as through the compilation and review of data required for product registration.
Throughout all project phases, including customisation according to individual design requirements, human factors studies, clinical supply and regulatory filing, all the necessary competencies for successful combination product development are centred at Ypsomed’s headquarters in Burgdorf (Switzerland). This enables an efficient dialogue among experts, supports rapid learning processes across specialist fields and helps to both establish and sustain effective risk management programmes.
BUILDING A GLOBAL MANUFACTURING NETWORK

Figure 1: The current Schwerin (Germany) site expansion will increase manufacturing capacity to 500 million injection devices annually.
Over the last 10 years, Ypsomed has manufactured more than 25 million reusable pen injectors and one billion prefilled pen injectors, and has supplied over 100 million prefilled autoinjectors since the first launch of YpsoMate in 2018. To accommodate the growing demand for increased production volume and new product launches, Ypsomed is expanding its manufacturing capacity in Switzerland, Germany (Figure 1), China and North America. Ypsomed’s two high-volume platform products, UnoPen and YpsoMate 1.0 mL, are currently manufactured at multiple sites, and this will be extended to the new sites in China and North America.
To free up manufacturing space to focus on core platform products, Ypsomed has announced the discontinuation of existing contract manufacturing and pen needle manufacturing at its large manufacturing site in Solothurn (Switzerland). Additionally, Ypsomed’s in-house toolmaking located in Burgdorf will be mirrored at the Solothurn site over the next 1–2 years. Higher cavity tooling and larger subassembly lines are being installed to increase manufacturing efficiency. This is reflected in the annual investments in fixed assets of CHF 120 million (£107 million) in the 2023/24 business year, which will increase to a total of CHF 1,500 million for the next five years.
The ability to supply platform products from multiple sites globally significantly reduces risks for Ypsomed’s pharma partners and their largest drug franchises. Furthermore, manufacturing close to the end market is more efficient and sustainable.
SERVING ALL CUSTOMERS
“YpsoMate autoinjectors have now been launched for 20 different branded drug products, with growing numbers of launches anticipated over the coming years.”
Ypsomed serves all therapy areas and customer segments with its platform products. Each customised product version is therefore supplied on a nonexclusive basis. Shared development and manufacturing infrastructure and resources benefit all customers. Today, over 70 separate drug products based on Ypsomed platforms are on the market around the world (Figure 2), with a further 150 projects moving through clinical trials or being prepared for commercial launch. YpsoMate autoinjectors have now been launched for 20 different branded drug products, with growing numbers of launches anticipated over the coming years. The largest number of these projects are in the autoinjector space for treating autoimmune and rare diseases, followed by pen injectors and autoinjectors for the insulin and GLP-1 franchises.

Figure 2: Ypsomed has over 70 products launched, and growing, serving all therapy areas.
For projects serving high-volume commercial quantities, Ypsomed supplies initial volumes through its shared platform product infrastructure and expands its manufacturing capacities in line with customer forecasts. Dedicated manufacturing lines for individual customers are also considered for the largest drug franchises. In order to reduce dependencies for such franchises, third-party contract manufacturing may also be considered under a royalty scheme.
40 YEARS OF STRATEGIC NETWORK PARTNERSHIPS – EXTENDING THE PLATFORM BEYOND THE DEVICE
For 40 years, Ypsomed has been building up a network of trusted strategic partners that, just like Ypsomed, serve as key suppliers to pharma customers. Over the last 20 years, these relationships have moved from cartridge-based peptide therapies for injection pens to include syringe-based autoinjector therapies to serve pharma and patient needs for antibody-based therapies. While Ypsomed strives to be best in class in the development and manufacture of self-injection devices, it partners with key suppliers to make sure that the drug-device combination product is supplied fast and on time and fulfils both pharma and patient needs.
By nurturing these strategic partnerships, pharma customers profit from best-in-class systems that lead to reduced costs and shorter timelines during the clinical, approval and commercial phases for each drug product. A recent example is how Ypsomed has entered into a strategic partnership with Interface Analysis Associates LLC (CA, US), a leading human factors services provider with long-standing expertise in supporting the pharmaceutical industry along the full combination product development journey.
PRIMARY CONTAINER COMPATIBILITY SPECIALIST
The starting point for every injectable drug-device combination product is the primary drug container – the cartridge or prefilled syringe. These containers are typically glass, although some may be polymer-based, but only function as a container system in combination with the elastomeric components that seal the drug in the container, such as a bilayer combi-seal, needle shield or plunger.
Understanding the primary drug container system and its interfaces with the injection device are key. Ypsomed collaborates and consults with both its customers and involved networking partners throughout the whole project to ensure that the selected drug container is fully compatible with the Ypsomed platform product whether the device is a pen injector, autoinjector or patch injector (Figure 3).
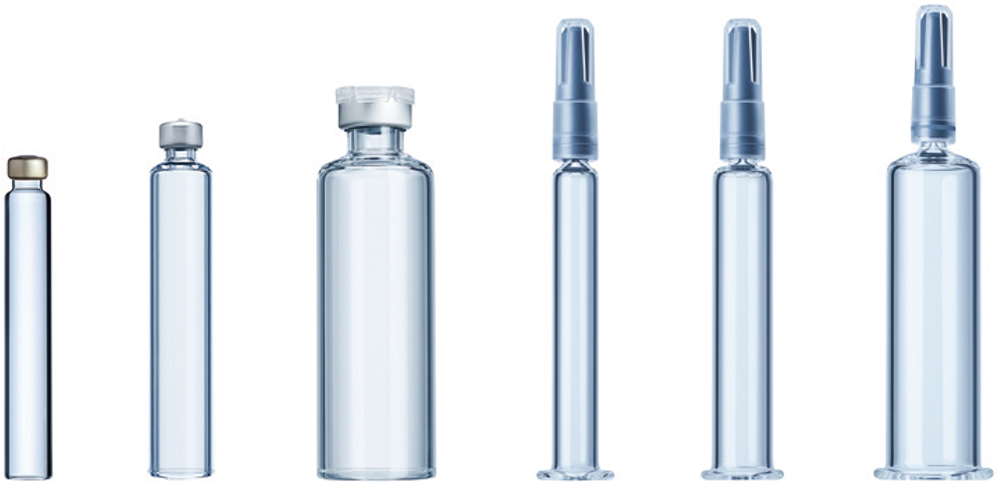
Figure 3: The main primary drug containers used in combination with Ypsomed’s platform products.
Ypsomed collaborates with over 15 different glass and elastomer manufacturers worldwide and tests the container variant prior to and during each and every project. When a primary drug container does not yet exist for a new platform injector, Ypsomed collaborates with leading suppliers to define and develop new industry standards, as is the case for the company’s recent platform product developments – YpsoMate 5.5 mL and YpsoDose 10 mL.
FINAL ASSEMBLY EQUIPMENT AND CDMOs
Final assembly of the drug with the prefilled pen injector, autoinjector or patch injector device is fundamental to the success of drug-device combination products. The development of easy-to-assemble platform products led Ypsomed to collaborate with a group of leading assembly equipment manufacturers (Figure 4). These companies supply scalable assembly solutions for both clinical and commercial supply based on clearly specified ready-toorder equipment. This extends to process aspects such as functional release testing, labelling and final packaging.

Figure 4: Ypsomed’s strategic partner network includes all leading final assembly equipment suppliers.
“When a pharma customer is considering final assembly, Ypsomed is at hand to make recommendations and facilitate the selection of manufacturer based on its established global network of fill-finish and final assembly CDMOs.”
When a pharma customer is considering final assembly, Ypsomed is on hand to make recommendations and facilitate the selection of manufacturer based on its established global network of fill finish and final assembly CDMOs. For YpsoDose, Ypsomed not only collaborates with SCHOTT Pharma for the 10 mL cartridge, but also with ten23 health to harness its formulation and process development, filling, assembly and testing of the final product.
FILL-FINISH AND OTHER NETWORKING SPECIALISTS
Understanding the cartridge or prefilled syringe dimensions is important, but understanding the filling process and the filled container as a system are critical to completing successful ISO verification testing for each combination product. Ypsomed collaborates with sterile filling equipment manufacturers to keep up to date with the latest filling process developments. Their equipment is used by both pharma’s in-house manufacturing and the global network of CDMOs that are involved in more than 50% of all pharma projects. With over 130 active customers, Ypsomed is a proactive and fully integrated player in the global pharma and CDMO manufacturing network.
Whether pharma customers are looking to establish contacts within the global drug-device consulting network or to source trainer devices or other specialist know-how, Ypsomed is always available to make recommendations from its extensive partner network.
Figure 5: Ypsomed’s comprehensive portfolio of platform products.
STRATEGIC NETWORKING IS PART OF YPSOMED’S DNA
Drug-device development and manufacturing is complex and more demanding than simply understanding the device itself. Ypsomed has been at the heart of the combination product ecosystem for over 40 years. The company’s comprehensive pen injector and autoinjector platform portfolio, including UnoPen and YpsoMate (Figure 5), and its partner network are ideally positioned to support the rapidly increasing demand for self-injection devices.
Learn more about Ypsomed, here.

