To Issue 166
Citation: Esseghir N, Fontayne A, Livemount L, “The Performance of Euroject – a BFS-Based Injection Device”. ONdrugDelivery, Issue 166 (Oct 2024), pp 70–72.
Nourhène Esseghir, Alexandre Fontayne and Laury Livemont discuss the performance and benefits of Unither‘s Euroject® injection system.
Injection systems available on the market, such as solutions for reconstitution in vials, prefilled syringes (PFSs) and multidose vials, have been used for decades. Unither designed Euroject® in a bid to revolutionise injectable products. It is a blow-fill-seal (BFS)-based prefilled single-dose vial injection system that allows direct administration without withdrawing the solution and adjusting the volume before injection, reducing the risk of errors.
“The fully automated and aseptic BFS process allows the production of enormous numbers of units within a short period of time.”
BENEFITS OF BFS TECHNOLOGY
BFS technology ensures continuous fill-finish of therapeutics in polymer unit doses for single use. The fully automated and aseptic BFS process allows the production of enormous numbers of units within a short period of time. Unither has a current production capacity of five billion doses per year. This manufacturing technology is a cost-effective fill-finish solution compared with traditional manufacturing methods for producing multidose glass vials and PFSs. BFS technology significantly enhances access to healthcare, especially in low- and middle-income countries. This innovation helps to meet high demand quickly and increases the affordability of vaccines and injectables, improving global health outcomes.
INNOVATIVE INJECTION DEVICE
The innovative Euroject injection device is a ready-to-use, preservative-free injection system that is easy to handle, efficient and does not require any lubricant, such as polytetrafluoroethylene (PTFE). Additionally, Euroject maintains strong competitiveness in its cost of goods sold (COGS), eliminating the 5–10% overfill necessary for multidose products. Furthermore, healthcare labour time is reduced because no manipulation is needed, as it is a ready-to-use, prefilled single-dose vial.
It is worth noting that the lightweight and compact nature of plastic containers from BFS reduces the likelihood of breakages, simplifying both transportation and storage. This contributes to a reduction in the carbon footprint. In addition, the BFS plastic container is made up of a recyclable mono-material and has a positive impact on the freezing time, allowing the optimisation of the cold chain.
ASSESSMENT OF EUROJECT DEVICE
To assess Euroject’s usability and injection performance, an evaluation of the system was carried out to determine multiple parameters, such as ease of the prefilled single-dose opening, connection tightness of the different components of the device, injection angle, delivery time and dead space (Figure 1). The results were analysed with reference to the different existing guidelines.
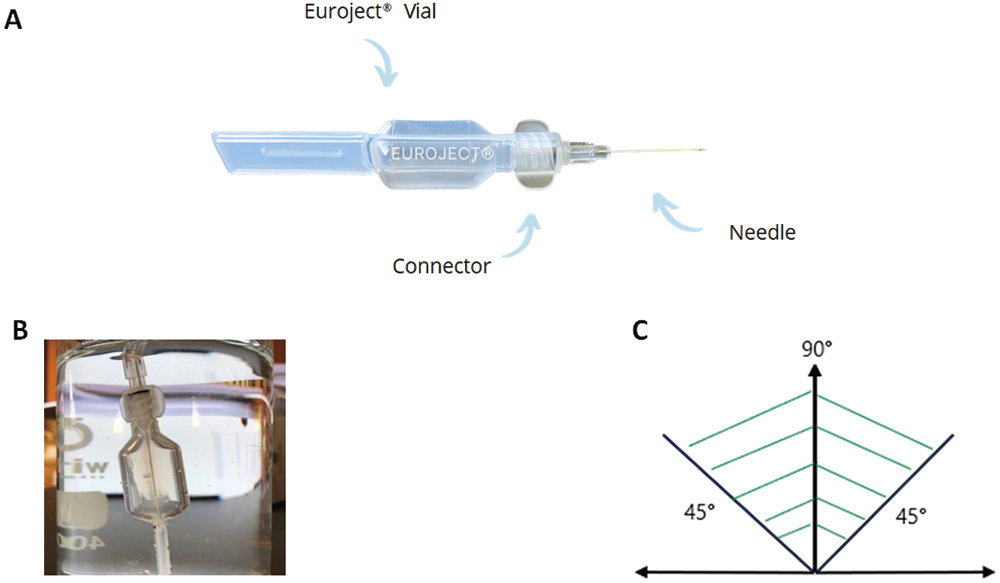
Figure 1: The Euroject injection device and its different components (A). The experimental set-up to evaluate the tight connection between the Euroject vial and the connector (B). The 45–90° injection angle, from the horizontal, allows for optimal performance of the Euroject injection device (C).
Opening and Connection Tightness
The BFS prefilled single-dose vial opening was conducted with a torque meter and resulted in an opening torque that varied between 13 and 17 Ncm. This range agrees with the force necessary to open prefilled, single-dose BFS vials that are commercially available for other medicines, such as eye drops.
The tightness of connection between the BFS prefilled single-dose vial and the connector was evaluated by an overpressure test, according to ISO 80369. All tests were compliant; the connection between the Euroject BFS prefilled single-dose vial and the connector was tight while applying a pressure of 3 bar to the system. This test confirms that the device can be used for direct injection and that the product will be delivered in its entirety to the patient once the Euroject is squeezed.
Usability Evaluation
The optimal usage of Euroject was assessed to achieve the best performance when administering the injection from the prefilled single-dose vial by varying the administration angle to the horizontal. As a result, the injected volume into the air or a skin pad was not impacted when the angle was between 45° and 90°. These results diverge from the traditional injection technique, which allows for horizontal syringe applications, such as vaccinations administered in the arm.
To ensure that this method was not a limitation, a preliminary survey was conducted with healthcare professionals. Two nurses and six physicians participated in the survey; all of them had experience practising medicine and performing vaccinations in low-income countries. The participants were given an opportunity to test the prototype device after reviewing the provided instruction leaflet. Subsequently, the injection test was divided into two segments for usability testing:
- Preparation of the injection device
- Injection using the injection device.
The device was received positively by the participants, as they found the device to be user friendly, fast and efficient, deeming it both functional and useful. Moreover, the new method of administration was not seen as a challenge in their practice.
Performance Evaluation
In the next step, the Euroject device’s performance was evaluated, with more than 3,800 BFS prefilled single-dose vials used during the study. The impact of the injection system (needle-connector) using different rings for a tight connection and different gauges in length and diameter (22G 1.5″; 25G 5/8″; 25G 1″) for different types of injection (intramuscular, subcutaneous and intradermal) was assessed.
A force of 15N was used, as recommended by the Program for Appropriate Technology in Health (PATH) for repetitive injections, so that the majority of users can deliver the intended prefilled single dose and ensure patient safety. Independently of the ring and of the needle size, the delivered volume was measured at 0.43 mL with a 95% confidence interval (CI) (0.428–0.430 mL) and a total loss of 0.06 mL. These results confirmed that the Euroject system is compliant with the ISO 7886–1 standard for dead volume.
Interestingly, with the Euroject injection system, the total loss in the system was 0.06 mL. This amount of loss includes what was left in the vial and in the needle. Since the Euroject system uses air to deliver the solution and not a plunger, the solution that remains in the needle is nearly zero, and the loss or dead volume corresponds mainly to the solution that remains on the vial’s walls. Therefore, the Euroject injection system is completely in line with WHO guidelines for HIV and hepatitis C (HCV) prevention, thanks to low dead space syringe and needles (LDSS/N). It is hypothesised that LDSS can reduce HIV and HCV transmission risk, as experiments showed that the volume of the residual fluid (blood) in the syringe following injection is a key factor in the survival of these viruses in syringes.
Delivery Time
The time needed to empty the Euroject injection system was also assessed and recorded during trials. Statistical analyses showed that needle size impacts delivery time. Indeed, in the experimental set-up, a needle hub with a 22G 1″ needle allowed the delivery of the entire volume in two seconds (Table 1). This durationis directly proportional to the length and indirectly proportional to the diameter. However, neither the injection pad, speed of the injection, injection force nor any other parameter impacted the delivered volume.
| 22G 1.5″ | 25G 5/8″ | 25G 1″ | |
| Injection angle | 90–45° | 90–45° | 90–45° |
| Delivered volume (mL) | 0.43 | 0.43 | 0.43 |
| Total loss (mL) | 0.06 | 0.06 | 0.06 |
| Delivery time (s) | 2 | 4 | 6 |
Table 1: Main performance attributes of the Euroject injection device.
“Across all the tests performed with Euroject… it was demonstrated that Unither’s device is compliant with the standards and guidelines set forth for injection devices.”
Across all the tests performed with Euroject, using a volume of water mimicking the most represented volume in intramuscular vaccine injection, it was demonstrated that Unither’s device is compliant with the standards and guidelines set forth for injection devices.
CONCLUSION
In summary, Euroject is a BFS-based, prefilled single-dose vial that has the following compelling properties, among others:
- Competitive COGS
- High production rate
- No risk of breakage during transport or administration
- Reduced transport weight
- Easy to handle
- No risk of dose accuracy
- No preservatives and additives, such as lubricant
- Compatible with all standard safety caps.
Considering various viscosities and dosages of medications, Unither and its development teams can assist pharmaceutical and biotech companies in assessing and modifying Euroject to suit their production needs and for use in therapy and vaccine applications.

