To Issue 166
Citation: “Company Showcase: PCI Pharma Services”. ONdrugDelivery, Issue 166 (Oct 2024), pp 112–114.
ADVANCED DRUG DELIVERY SOLUTIONS
As a trusted partner, PCI Pharma Services offers expertise and solutions in sterile fill-finish and specialist final assembly and packaging of advanced drug delivery and drug-device combination products. The company’s seamless end-to-end injectable solutions, comprehensive approach and dedication to excellence positions it as a leader in optimising dosing and providing convenient, easy-to-use, patient-centric therapies to improve the lives of patients.
One of PCI’s key strengths is the flexibility it provides in offering solutions that cater to a diverse global client base. Whether manufacturing or packaging niche personalised medicines or large-annual-volume treatments, it has the capability and capacity to scale its services to meet specific needs, delivering streamlined supply chain solutions.
Sterile Fill-Finish and Lyophilisation
PCI’s scalable global manufacturing capabilities in sterile formulations and lyophilisation cover a broad range of small and large molecules, such as monoclonal antibodies, oligonucleotides and peptide drug products across multiple delivery formats, including vials, prefilled syringes (PFSs), autoinjectors, on-body injectors (OBIs) and cartridges for pens (Figure 1).
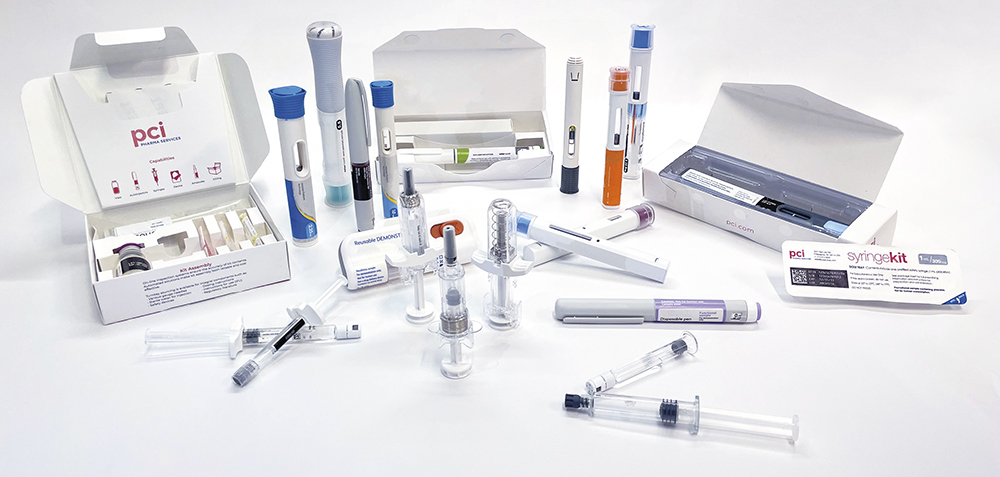
Figure 1: PCI’s expertise covers a wide range of formulations and devices.
Advanced Drug Delivery and Combination Products
Driven by innovation and patient centricity, PCI’s design and development expertise, combined with its device-agnostic assembly and packaging capabilities, offer flexible solutions for a diverse portfolio of conventional and specialty injectable drug delivery devices. Tailored to unique design, development and manufacturing needs, PCI offers a complete range of capabilities, services and expertise, including:
- Expert design processes focused on human-factors engineering and technical functionality
- Optimising packaging designs for manufacturability, scalability, automation and sustainability
- End-to-end drug-device combination services, including:
– PFS assembly
– Pen, autoinjector and OBI final assembly
– Needle safety syringe assembly and precision labelling
– Integrated side- or top-open cartoning
– In-process functional testing and final release
– Serialisation
– Cold chain storage.
FROM DEVICE STRATEGY TO PACKAGING DESIGN & SUSTAINABILITY
With a global network of experts, PCI can provide guidance at critical time points to assist in the development of patient-centric drug device combination products. The company’s wealth of industry experience can help to determine the best device container and strategy for a drug product and patient population – from the use of established, well-accepted platforms with regulatory approval to a more innovative device approach that may be more attractive for specific patient populations than more traditional platforms.
Figure 2: PCI’s service offering includes drug-device combination product assembly, packaging and labelling.
PCI’s pharmaceutical packaging design department provides an innovative and value-added service (Figure 2). The company’s dedicated team of in-house design specialists can deliver insightful packaging design and practical knowledge to deliver differentiated, sustainable and cost-effective packaging solutions. Working with its partners from as early as possible, the design department and a crossfunctional network of experts in sterile drug product manufacturing, engineering, operations and approved vendors are able to develop expert design processes focused on human factors engineering and technical functionality, delivering designs optimised for manufacturability, scalability and automation. This seamless solution ensures that key considerations are addressed at the right time, leading to both cost and time efficiencies and ultimately ensuring speed to market.
SCALABLE CLINICAL TO COMMERCIAL SUPPLY
With customer focus and flexibility at the core of PCI’s drug-device assembly and packaging capabilities, the company’s device-agnostic technologies can adapt to the unique requirements of varying platforms and bespoke device requirements, from concept to commercialisation. For example, PCI’s specialised clinical and low-volume commercial autoinjector assembly line provides a multi-platform autoinjector solution as well as the capability to assemble and label needle safety device platforms, making it the ideal technology for development studies, clinical trials and niche orphan drugs.
Providing an integrated, scalable solution, PCI’s mid-to-high volume commercial assembly technologies are also able to accommodate multiple drug device combination product types at a larger scale for later-stage clinical programmes, product launches and ongoing commercial market supply. PCI’s technologies can easily and cost-effectively be tooled for new autoinjector parts, enabling product customisation and allowing PCI to respond quickly and efficiently to technological changes and innovations.
SUPPORTING THE DELIVERY OF LIFE-CHANGING THERAPIES
Sterile Fill-Finish and Lyophilisation Expansion
With a US$100 million (£76 million) investment, PCI is expanding its established sterile development and manufacturing campus in Bedford (NH, US) with a new multi-product, 50,000 sq ft facility. The new facility will provide best-in-class latestage clinical and commercial capacity, featuring Annex 1 compliant technology, including an aseptic fill-finish line within a fully isolated containment system, complete with twin 40 m2 lyophilisers with automatic loading and unloading systems. Processing batch sizes up to 300,000 vials at a rate of 400 vials per minute, this investment provides much needed large-scale capacity for the filling of life-changing small and large molecules, including biologics.
Expansion of Drug-Device Capabilities and Capacities
Complementing the continued growth and investment across its sterile manufacturing network and part of its global investment plan to address growing client demand for innovative injectable packaging solutions and readily available scalable capacity, PCI is investing more than $365 million in facilities and infrastructure supporting the clinical and commercial final assembly and packaging of advanced drug delivery systems, with an emphasis on drug-device injectable formats.
Ireland: Facility Acquisition and Footprint Expansion
A key highlight of PCI’s ongoing expansion initiative is the acquisition of a purpose built pharmaceutical packaging facility in Dundalk, Ireland. The 90,000 sq ft facility is set to begin operations in Q4 2024 and will offer scalable drug device assembly and packaging for both injectable and oral solid-dose drug products. The site will also feature 3,000 pallet spaces of dedicated controlled ambient storage and 2,500 pallet spaces of 2–8°C refrigerated storage capacity to support its operations.
In addition, PCI has commenced construction on a new 80,000 sq ft packaging facility at its CityNorth campus in Stamullen, Ireland. Expected to be operational by Q3 2025, this plant will expand capacity for the final assembly, labelling and packaging of injectable drug products, including vials, PFSs, and drugdevice combination products, including autoinjectors. The facility will also add 500 2–8°C pallet spaces and an additional 4,000 ambient pallet spaces, providing enhanced temperature-controlled storage capacity for the site.
Rockford: Clinical and Commercial Expansion
Building upon its specialised injectable packaging solutions at PCI Rockford (IL, US), PCI is in the final stages of completing a new 475,000 sq ft facility to meet the growing market need of specialised assembly and packaging for injectable drug-device combination products. Over 20 dedicated suites will support the final assembly and packaging of vials, PFSs, autoinjectors, OBIs and pen injectors, such as those for the treatment of diabetes and obesity, in addition to oncology and autoimmune diseases.
With the aim of accelerating advanced drug delivery and drug-device combination products through clinical trials to commercialisation as efficiently and costeffectively as possible, PCI is expanding its Rockford campus further with an additional 70,000 sq ft facility. This facility will support the whole development lifespan of injectable drug products under one roof, from clinical to commercial, marrying efficiencies and streamlining drug product journeys.
SEAMLESS, ACCELERATED SUPPLY CHAIN SOLUTIONS
As an integrated global contract development and manufacturing organisation, PCI is a manufacturing, packaging and supply chain expert able to provide seamless sterile fill-finish and lyophilisation solutions from development to commercialisation. Additionally, PCI offers integrated custom packaging solutions for sterile injectables that enable knowledge sharing and communication between teams to ensure that drugdevice combination product packaging is optimised for the product, patient and production.
Supporting a quick-to-patient supply chain, PCI’s scalable device-agnostic technologies, coupled with its extensive, readily available assembly and test tooling for the most common device platforms, removes the lengthy lead time of up to six months for new tool sets and provides significant validation time and asset cost savings.
With in-house laboratories, PCI provides a range of packaging and analytical services to support development, clinical and commercial supply of medicines globally. From product ID testing, method transfer, release and stability testing to autoinjector system testing with functional tests and ISO 11608 standards to assess key performance metrics, such as cap removal force, activation force, dose accuracy and injection time, PCI can ensure that its partners’ therapies meet regulatory guidelines and are safe for patient use.
PCI is undertaking a significant expansion of its capacity and capabilities and, as a trusted partner, provides expertise and solutions tailored to the unique demands of drug development programmes and the patients that they serve. The company’s comprehensive approach and dedication to excellence in delivering customer service positions it as a leader in this rapidly evolving industry (Figure 3).
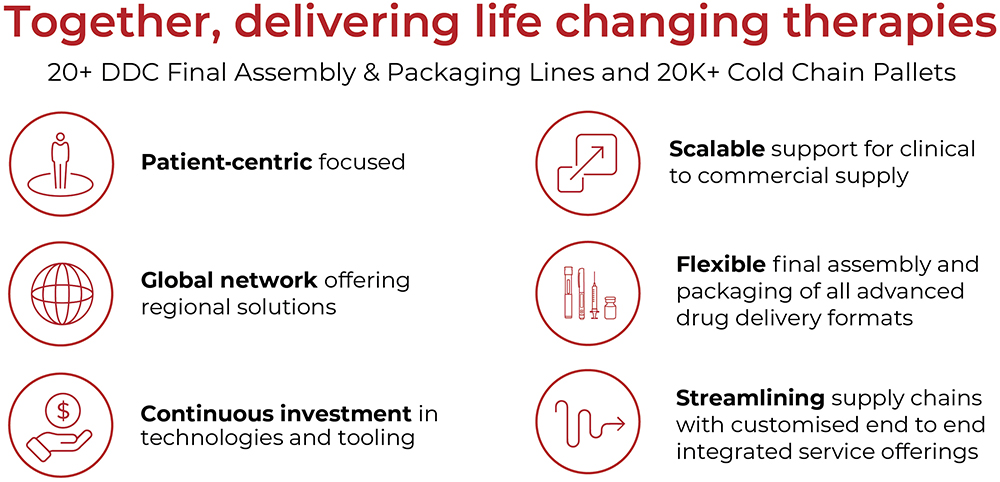
Figure 3: The advantages of partnering with PCI.

