To Issue 167
Citation: Storz J, Mueller G, “Interview: PureHale® and the Future of Fine Mist Dispensers for Upper Airway Treatments”. ONdrugDelivery, Issue 167 (Nov 2024), pp 22–26.
Q What are the most common upper airway conditions and how are they currently being treated?
JS First, we should define the upper airway – it consists of the nasal cavity, oral cavity, pharynx and larynx. Together, they serve as the major pathway for air to enter the body and consequently constitute a primary interface with the environment. The upper airway is important to human health, as it can be an entry point for contaminants or disease. However, it can also serve as a place for healing and protection. Some of the most common conditions that affect this sensitive pathway include allergies, sinusitis, dry mucosa and dry nose, as well as viral and bacterial infections. Most of these conditions can cause inflammation of the mucosal tissue in the upper airways, which can have a significant impact on an individual’s health and wellbeing. As a result, there is a demand for products that can prevent or treat these conditions, which range from irritating to debilitating.

Figure 1: Airway disorders, including coughs, colds, allergies and asthma, can be treated using a range of technologies, including nasal sprays, aerosol dispensers, throat sprays, nebulisers and inhalers.
There are several different technology solutions to deliver formulations that can soothe, relieve or eliminate symptoms, but, to directly treat a target area, a technology system is needed that can reliably isolate delivery of the product to the desired region of the respiratory system. There is a range of different technologies available that can deliver treatments to different regions of the respiratory system, some of which are more targeted than others. For example, a mechanism that targets product delivery to the upper airway while minimising the amount of product reaching beyond it, such as the lower airway or lungs. Some of the devices used to treat upper airway conditions include sprays, droppers, rinsing devices, squeeze bottles, aerosol dispensers, inhalers, nebulisers and throat sprays (Figure 1), not all of which target the upper airways. For example, nebulisers deliver mist to the lungs, even though it passes through the upper airway. The droplet size of a fine mist can impact deposition rates in different regions of the respiratory system. Therefore, controlling droplet size from a fine mist delivery system can effectively focus delivery to the desired region, maximising the benefit for the consumer. These complex technical considerations must be made when designing new products to treat upper airway conditions.
Q What are the trends in upper airway treatments from the consumer’s perspective?
JS There is a wide range of consumers looking for upper airway treatments. For example, we have seen growing interest from parents seeking convenient treatments for their infants and toddlers, as well as user-friendly products for seniors. In part, this is being driven by increasing consumer demand for more natural, non-chemical drug treatments. Consumers are seeking products that can deliver relieving, cleansing, moisturising and soothing effects to the upper airways, as well as prophylactic inflammation and symptomatic treatments. Consumers have also expressed a preference for products that are non-invasive and avoid harsh rinsing steps. The ease-of-use and natural composition of some upper airway products, such as sterile saline fine mists, have made them suitable for moisturising and soothing the upper respiratory systems of babies, toddlers and children.
A 2021 study1 found that regular, daily use of saline nasal sprays or drops could provide relief from nasal symptoms in children and adults suffering from upper respiratory tract infections or allergic rhinitis with no reported side effects. This finding aligns with national guidance recommending daily use of saline sprays or drops for improving daily nasal hygiene. Another more recent study demonstrated that using saline nasal sprays at the first signs of a respiratory infection shortened illness duration by as much as three days.2 So, there is evidence that upper airway saline treatments can provide measurable benefits for consumers. And, since the covid-19 pandemic, there has been growing interest in upper airway products for preventative applications instead of the more common acute symptom relief or treatment. Overall, we believe that consumers are looking for a portable and non-invasive upper airway delivery technology that is easy to use and can deliver a wide range of natural products to consumers of virtually any age. Aptar Pharma’s PureHale® system was designed with many of the features required to address today’s upper respiratory ailments.

Figure 2: (A) Aptar Pharma’s PureHale® system comes with a face mask and mouthpiece, suitable for a variety of users and for nasal and mouth application. (B) Aptar Pharma’s PureHale® system produces a continuous plume of fine mist, reaching the upper airways based on the defined range of droplet size.
Q What is Aptar Pharma’s PureHale® and how does it work?
GM Aptar Pharma’s PureHale® system was specifically designed to deliver a fine mist to the upper airways in a portable and user-friendly solution (Figure 2A). University of Cyprus simulation studies3 have demonstrated that PureHale® represents a technical advancement in targeted upper airway delivery that aligns well with consumer needs. Aptar Pharma developed PureHale® with an interchangeable mask and mouthpiece option (Figure 2B) that the the system can meet varied user requirements. PureHale® is based on well-known Bag-On-Valve (BOV) technology, using only compressed air to drive the release of a fine mist for up to 90 minutes of continuous use. Typical nasal sprays can only reach the frontal part of the nasal cavity with their spray mist. However, PureHale® can reach all the way back to the rear of the nasal cavity, also known as the nasal associated lymphoid tissues or “NALT” region. This provides wider delivery options to the upper airways. The ability to deliver fine mist saline formulations to these regions can contribute to improved mucociliary clearance and general nasal hygiene.
Q Does Aptar Pharma have data supporting the performance of its PureHale® fine mist dispenser?
JS When Aptar Pharma designed PureHale® for upper airway applications, we performed various tests to ensure that it would deliver a fine mist with defined droplet sizes to specifically target the upper airway. Our scientists, in conjunction with researchers from the University of Cyprus,3 studied the deposition of its fine mist, via both its mask and mouthpiece configurations, to demonstrate the quantity of product delivered to the targeted regions (Figure 3). It was determined that a consistent droplet size range of 15–20 μm is ideal for targeting product deposition to the upper airways without impacting the lungs or lower airways. We used aerodynamic particle size distribution testing using a next-generation pharmaceutical impactor in combination with an Alberta Idealised Throat to simulate deposition and determine droplet size distributions in the upper airways. Combining this with simulated breathing models and computational fluid particle dynamics allowed our scientists to map the droplet deposition accurately throughout the regions of the upper airways.
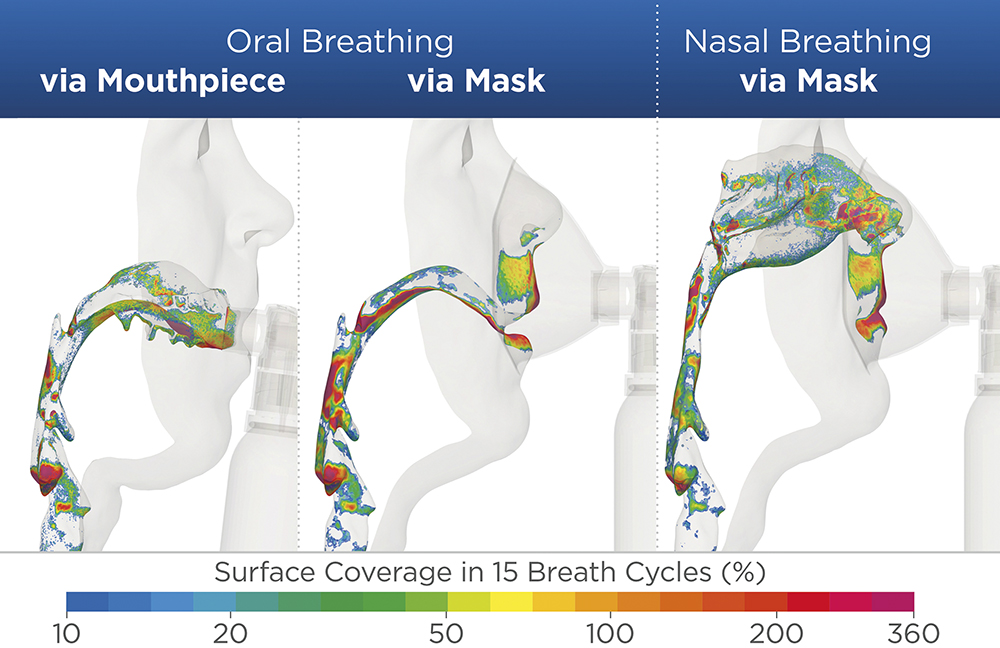
Figure 3: Estimated airway surface coverage after 15 breathing cycles for all three available PureHale® usage configurations.
“When the PureHale® aerosol plume was aimed directly at the airways, it delivered high deposition rates in the targeted upper airway regions.”
We also assessed the deposition patterns in adjacent regions of the respiratory system to ensure that the product is only delivered where it is needed. The results found that, when the PureHale® aerosol plume was aimed directly at the airways, it delivered high deposition rates in the targeted upper airway regions. When an additional breathing manoeuvre was performed by the user, separating inhalation (mouth) and exhalation (nose), the resulting data showed that upper airway deposition could be even further increased. The data-driven development of PureHale® confirmed that it can reliably deliver appropriately sized droplets of product to targeted areas of the upper airways.

Figure 4: Summary of the most common key consumer feedback comments regarding PureHale®-based saline products found on major US market e-commerce channels (data on file).
Q Why is the PureHale® fine mist dispenser attractive to consumers?
GM PureHale® was designed to be portable and user-friendly. A simple turn of the actuator ring releases a constant flow of fine mist, typically used in 1–2-minute applications. The system fits in most handbags and provides silent operation for on-the-go applications. This portability is possible because PureHale® is driven by compressed air only, requiring no wires or external power sources. As a result, the system can be administered virtually anywhere and emits the same fine mist in any position, from lying down to standing up. Even with its mask attached, the assembled product was designed to remain standing upright for consumer convenience. PureHale® is designed to be used daily for preventative or maintenance treatments or, occasionally, for symptom relief. We believe that consumers are quickly adopting products that use PureHale® technology for many of these important reasons. In 2023 alone, US consumers spent over US$40 million (£30 million) on products that use the PureHale® system (Figure 4).

Figure 5: Infant using Aptar Pharma’s PureHale® system with mask configuration.
Q Why do parents of infants and toddlers like to use the PureHale® system?
JS The US FDA does not recommend the use of over-the-counter cough and cold medications for children under the age of four, so parents are seeking non-medicated alternatives. There is also increased awareness of the benefits of fine mist saline products for infants, with significant sales growth forecast in North America and Europe, as noted in a recent study, “Baby Saline Spray Market Research Report 2024”, published by Verified Market Reports. Parents also like the fact that fine mist products are non-invasive, which increases patient acceptance. This is particularly important for children when they are not feeling their best. The fine, comforting, cool mist generated by PureHale® systems is silent and friendly to even the smallest nostril size. This makes administration of the product easier for parents, while reducing the child’s fears (Figure 5). Parents also appreciate that the mask and mouthpiece are easy to clean and reuseable, so PureHale® is always ready to go. A review of parent customer comments on US-based online retailers commonly highlight the ease of administration and lack of resistance to their use from children as important features of the PureHale® system.
Q Do you see additional new applications for the PureHale® system?
JS We do see additional potential applications for PureHale® beyond saline products. We have received interest in applying the PureHale® platform to scented products that refresh the user and their immediate surroundings. PureHale® delivers these scented mist products in a clean, hygienic and portable format, broadening their appeal. Similarly, in the personal care space, we anticipate more applications for personal care products, such as skin moisturisers, general skin care and refreshing consumer products presented as a fine mist.
While additional research would be required, we believe there is a potential for the use of fine mist formulations to treat upper airway areas that are associated with virus adherence and replication.
“Through its specialised laboratories, Aptar Pharma can also perform additional specific tests based on the individual formulation’s requirements.”
Q How does Aptar Pharma support customers who select PureHale® to deliver their fine mist products?
GM We support customers in their development work using specialised analytical tests, models and simulations to ensure customer formulations are compatible with PureHale® technology. This development work may include various one-time characterisation tests that are part of the system’s basic performance analysis, such as droplet size distribution, full-to-empty tests, spray pattern or flow rate. Through its specialised laboratories, Aptar Pharma can also perform additional specific tests based on the individual formulation’s requirements. Our experienced scientists can help customers to optimise their formulation for use with PureHale® further by providing them with technical feedback and suggestions that can help to accelerate the product development process. Ultimately, our goal is to support customers in developing a fine mist product that will meet their performance requirements throughout the life of the product.
Q Does Aptar Pharma also provide scale-up and manufacturing support for products using PureHale® systems?
GM Aptar Pharma has established long-standing relationships with several contract development and manufacturing organisations (CDMOs) located in different geographic regions that offer PureHale® filling capabilities that can support local markets or global supply. This potentially simplifies the manufacturing process for customers, as they can access CDMOs with both the right manufacturing capabilities alreadyin place and prior experience in filling PureHale® systems. Aptar Pharma can also provide additional laboratory data to support customer product development programmes. It is important that we support PureHale® customers at every step of the development process so that they can achieve long-term success.
Q Is PureHale® available in different configurations for different applications?
GM Our PureHale® system offers a range of customisation options to customers. The BOV systems come in three different bag size options holding 30, 50 and 85 mL of formulation. These offer approximately 30, 60 and 90 minutes of continuous use, respectively. Standard PureHale® mask and mouthpiece colours are transparent white and transparent blue. However, Aptar Pharma also offers branding options with mask and mouthpiece components available in a range of custom colours.
“Product companies are trying to meet changing consumer expectations for upper airway treatments with innovative and convenient solutions.”
Q Is PureHale® available globally and are there any regional trends in demand?
JS There are established market references using the PureHale® system found around the world, including the American, Chinese and Indian markets (Figure 6). The US market has seen continued interest in saline products as new, innovative products are being launched. We also expect to see continued growth in PureHale® products in Europe, where other nasal saline rinse treatments are already well accepted and popular. We support PureHale® customers globally, ranging from small local brands to multinational product companies. Product companies are trying to meet changing consumer expectations for upper airway treatments with innovative and convenient solutions. Overall, we are observing increased demand for our PureHalev system, driven by both product differentiation and consumer demand for upper airway delivery systems.
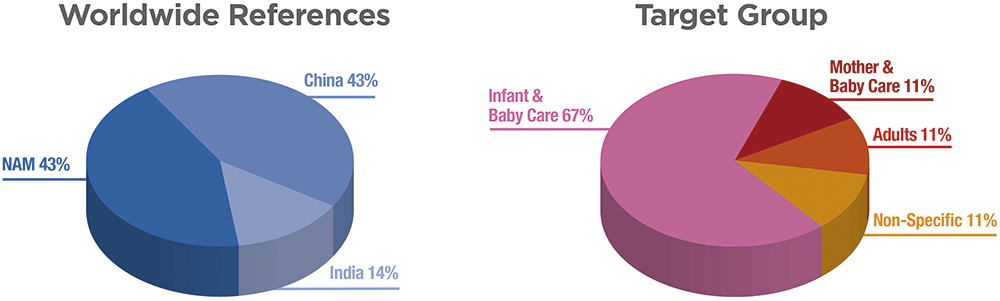
Figure 6: Graph of proportion of regional market references that use Aptar Pharma’s PureHale® system in North America, China and India. The majority of market references are targeting infant and baby-care applications.
To learn more about Aptar Pharma’s PureHale system, click here.
REFERENCES
- Santoro E, Kalita P, Novak P, “The Role of Saline Nasal Sprays or Drops in Nasal Hygiene: A Review of the Evidence and Clinical Perspectives”. Rhinology Online, 2021, Vol 4, pp 1–16.
- Little P et al, “ Nasal Sprays and Behavioural Interventions Compared with Usual Care For Acute Respiratory Illness In Primary Care: A Randomised, Controlled, Open-Label, Parallel-Group Trial.” Lancet Respir Med, 2024, Vol 12(8), pp 619–632.
- Marx D, Stylianou F, Kossinos S. “Aerosol Deposition Characterization of Innovative PureHale® Technology Targeting the Upper Airways”. Drug Development & Delivery, Sep 2021, Vol 21(6), pp 16–23.

