To Issue 168
Citation: Thueer T, Muenzer C, Manger S, “PiccoJect – Advancing Self-Injection of High-Viscosity Formulations”. ONdrugDelivery, Issue 168 (Jan 2025), pp 50–53.
Thomas Thueer, Chris Muenzer and Stefanie Manger discuss the challenges posed to drug delivery device designers by the needs of biologic therapies. In response, Haselmeier has developed the PiccoJect autoinjector, which has a novel parallel spring layout that enables a newfound flexibility in design, ideally positioning it to rise to the challenges of delivering high-viscosity biologics.
THE CONTINUED NEED FOR SELF-INJECTION
Since the early 2000s, the injectable route has been one of the most exciting and fastest-growing areas in drug delivery. Even with the recent interest in obesity medications, the rising number of complex therapies making their way through pharmaceutical pipelines over the last 20 years has been a primary driver of increasing interest in injectable therapies. In addition, there has also been a significant push towards administering therapies in patients’ homes rather than in clinical environments when possible. Doing so has numerous benefits, from increased convenience for patients to a reduced burden on healthcare systems and lower environmental impact from the need for patients to travel to and from a clinic.
Reflecting this interest, the global self-injection devices market was valued at US$22.8 billion (£17.9 billion) in a report by Grand View Research, with a projected compound annual growth rate of 10.3% from 2025–2030.1 With this growth in the market, it will be critical for the drug delivery industry to continue to innovate, making more patient-friendly devices and accommodating a wider array of therapies to meet demand.
However, delivering these molecules via injection, often in large volumes or highly viscous formulations, is not without its own set of challenges. This has led to a steady stream of innovation in the development of injection devices to facilitate the delivery of biologics and other novel therapeutics. Specialist drug delivery device developers have worked to find ways to effectively deliver increasingly difficult molecules, pushing injectable drug delivery in new directions, such as high-viscosity autoinjectors and wearable devices.
DELIVERING BIOLOGICS
The Challenges
Biologics are a notoriously difficult category of therapeutic to deliver. The molecules are large, often fragile and prone to agglomerate or denature under unfavourable conditions, drastically reducing their therapeutic effect. Challenges with delivery via the oral route, where the gastrointestinal tract presents a near-insurmountable barrier to the fragile molecules, have led drug developers to see injection as the natural home for biologics.
Additionally, new targets and newer technologies with lower bioavailability have resulted in an increase in dose size. This, in turn, has resulted in therapies that require the delivery of either high volumes or high viscosities.
High-viscosity drugs are particularly challenging to deliver. In order to minimise pain, and the knock-on effects to adherence, device designers tend to prefer to keep needle gauges as narrow as possible, which means much higher injection forces are required for delivery. Higher forces in turn require more power, such as larger, heavier springs or an alternative power source, all of which must fit in a compact device format. In the case of larger springs, these also place more strain on the device housing during storage, necessitating more robust construction and materials.
Not only is designing for high viscosity a technical challenge in and of itself, but it must also be done in the context of patient-centric design. Ultimately, the purpose of any drug delivery device is to administer the contained therapeutic to a patient and so must be designed with that patient in mind – especially in the case of at-home administration. If the medicine is to be self-administered successfully, the device must be approachable and intuitive, as well as convenient, for the patient to understand and use, often on a regular basis. If the device is unacceptable in some way, such as being too difficult to use or causing too much pain, patients may make errors during delivery or be inclined to discontinue their therapy, resulting in failed treatment and disease exacerbations that lead to patients needing hospital treatment and increased strain on healthcare systems.
Therefore, any new entrants into the self-injection market must meet the exacting demands of the biologics they aim to inject and offer patients an appealing user experience. As such, device designers have attempted a variety of approaches to achieve these twin objectives, continually innovating and improving on what is available with devices capable of delivering ever-higher volumes and viscosities to enable formulators to bring increasingly challenging therapeutics to market.
Current Approaches
Wearable injectors are a more recent addition to the drug delivery device landscape. On paper, the idea is straightforward – formulation viscosity can be reduced to more manageable levels by increasing formulation volume beyond that which autoinjectors can handle. Wearable injectors, which are usually attached to the body via an adhesive patch, can deliver their sizeable payloads over an extended period of time, circumventing some of the design challenges presented by high viscosity. While many of these devices have been developed, there have been limited commercial successes, which is likely due to many devices being incompatible with established filling equipment and the requirement that patients have to wear them for extended periods, meaning that they must be both comfortable and discrete to be acceptable compared with a regular injection. Furthermore, these devices present significant technical challenges to developers due to their inherent complexity, which also leads to an increase in cost.
On the other hand, high-viscosity autoinjectors are an iteration on existing, well-established and accepted technology. The first high-viscosity autoinjectors were, in essence, scaled-up versions of existing autoinjectors, with larger springs and more advanced drive systems, such as lead screw mechanisms, to increase their output force. Since the initial launch of these devices, high-viscosity autoinjectors based on electromechanical drive systems have been developed as an alternative. In both cases, the high-viscosity versions use a specialised drive system, which is more complex, meaning pharmaceutical companies with varied portfolios must use a different device for their high-viscosity and low-viscosity formulations. Additionally, it is important to bear in mind that increasing the complexity of the drive system leads to a higher cost for the final device.
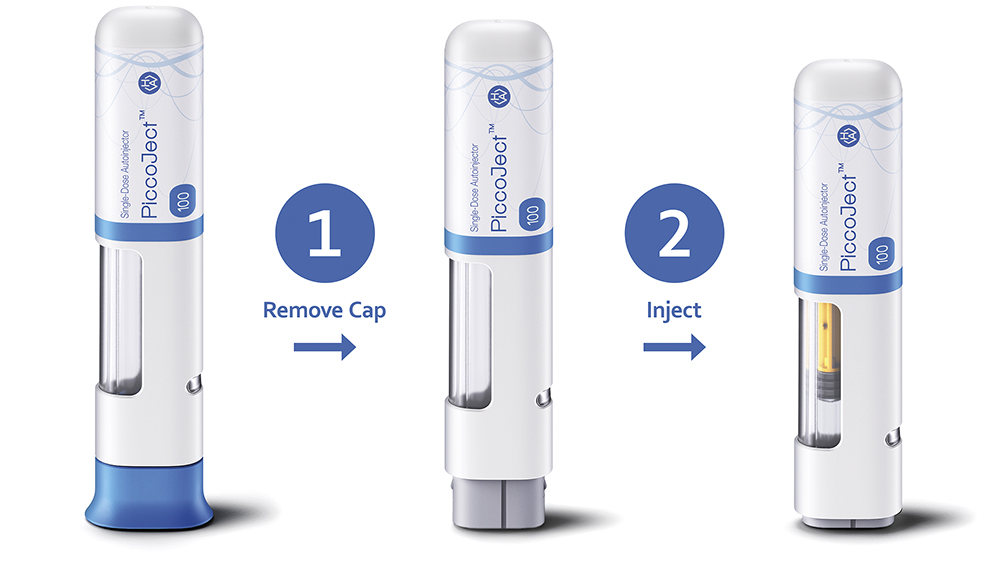
Figure 1: Haselmeier’s PiccoJect™ is a compact, fully-featured two-step autoinjector designed for subcutaneous delivery of drug products, compatible with any standard 1 mL long or 2.25 mL prefilled syringe.
These current approaches leave space in the market for a more elegant solution to the challenges posed by delivering biologics. A device that is capable of delivering a wide range of viscosities would provide pharmaceutical companies with an ideal solution and, by employing a parallel spring layout, Haselmeier’s PiccoJect autoinjector is able to provide exactly that (Figure 1).
“Haselmeier has taken a new approach to the layout of the drive mechanism within the device – by placing the spring parallel to the syringe, rather than in sequence behind the plunger, piccoject unlocks unprecedented flexibility in its design.”
PICCOJECT – AN INNOVATIVE PARALLEL SPRING LAYOUT
PiccoJect™ is a novel autoinjector capable of delivering both high- and low-viscosity formulations from a single device. To achieve this, Haselmeier has taken a new approach to the layout of the drive mechanism within the device – by placing the spring parallel to the syringe rather, than in sequence behind the plunger, PiccoJect unlocks unprecedented flexibility in its design. This parallel layout enables the delivery force to be fine-tuned to suit any given formulation by varying the spring to provide the required force. The critical advantage of PiccoJect’s parallel spring layout is that it enables greater variation in the diameter of the spring by moving it out of line with the syringe (Figure 2).

Figure 2: PiccoJect’s innovative parallel spring layout enables significant design flexibility, accommodating both low- and high-viscosity formulations within a single device.
When designing springs, a critical parameter to be aware of is the “spring index” – the ratio between the coil diameter and the wire diameter of the spring. This relationship provides valuable information about the stress and manufacturability of a spring. By not being constrained by the diameter of the syringe, PiccoJect’s layout enables the use of springs with larger diameters compared with what is possible with a traditional autoinjector. In turn, this allows the use of larger diameter spring wire while maintaining an optimal spring index. As such, PiccoJect can deliver a high dispense force with reduced material stress, manufacturing complexity and cost.
Naturally, PiccoJect’s parallel spring layout has a significant impact on the device’s shape. PiccoJect is shorter than traditional autoinjectors, with a wide cross-section rather than the narrow cylindrical one usually seen with co-axial layouts. Extensive human factors testing by Haselmeier has demonstrated that users show a preference for PiccoJect’s shape, which they have described as more comfortable. This alternative form factor also enables PiccoJect to have a larger, wraparound drug window for patients to track injection progress and perform visual drug inspection, which has also received high approval rates from users in Haselmeier’s human factors testing.2
Along with its patient-centric format and features, PiccoJect has been designed with the needs of pharmaceutical manufacturing in mind. PiccoJect’s parallel spring layout has been designed to be compatible with any standard 1 mL long or 2.25 mL prefilled syringe. The device itself is comprised of only eight parts, which can be manufactured using unfilled polymers, despite the large spring forces, making the device more sustainable.3
CONCLUSION
Today, self-injection devices are a critical component of the current drug delivery landscape. A combined need for patient-centric devices and the delivery of high-viscosity formulations has fuelled innovation in the sector, as device designers innovate to fulfil the unmet needs of patients and the industry.
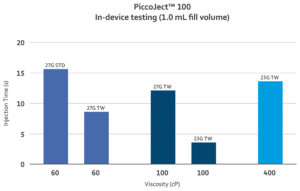
Figure 3: Test results from in-device testing of PiccoJect 100 with prefilled syringes containing 1 mL of highly viscous glycerol-based solutions (60, 100 and 400 cP) using various needle gauges.
Haselmeier has provided the next step in the pursuit of patient-friendly delivery of high-viscosity biologics. The PiccoJect autoinjector employs a novel parallel spring layout to offer numerous benefits to patients and pharmaceutical companies alike. For users, the parallel spring layout provides a shorter, wider form factor that human factors studies have shown is preferable for a wide array of users, as well as other patient-centric features such as the large, wraparound dose window. For pharmaceutical companies, PiccoJect’s parallel spring layout enables the drive mechanism to be tuned to the needs of a given formulation, making it suitable for both high and low viscosities (Figure 3).
The innovative layout of PiccoJect is a significant step forward in autoinjector design. Capable of handling a wide range of viscosities with the same drive mechanism, PiccoJect can offer pharmaceutical companies a single platform device for a vast range of biologic formulations. Comprising only eight parts, PiccoJect is simple, effective and reliable. The advantages unlocked by the parallel spring layout and patient-centric design are able to provide the foundation for the next generation of biologic drug delivery.
REFERENCES
- “Self-injection Devices Market Size, Share & Trends Analysis By Product, By Usability, By Application, By Region, And Segment Forecasts, 2025 – 2030”. Market Report, Grand View Research, 2024.
- Thueer T, Muenzer C, Schlenker I, “PiccoJect – Using Human Factors to Fulfil Patients’ Need for Injection Confidence”. ONdrugDelivery, Issue 152 (Oct 2023), pp 44–48.
- Metzmann F, Muenzer C, “Excellence Through Simplicity: PiccoJect Autoinjector Platform”. ONdrugDelivery, Issue 133 (May 2022), pp 63–68.

