To Issue 168
Citation: Yeh H-J, Lai J-R, Cheng T-C, “Novel Solutions for Ultra-Large-Volume Wearable and Portable Subcutaneous Injectors”. ONdrugDelivery, Issue 168 (Jan 2025), pp 77–83.
Dr Hong-Jun Yeh, Jiunn-Ru Lai and Tsung-Chieh Cheng discuss the need for novel approaches to the challenge of delivering high volumes of biologics while also addressing patient centricity, and introduce MicroMED’s two innovative gas-powered approaches that leverage micro-electromechanical systems to drive the injection.
INTRODUCTION
The subcutaneous (SC) drug delivery system landscape is rapidly evolving, driven by the increasing demand for higher-dose biotherapeutics and patient-centric considerations, such as increased treatment tolerability and quality of life. Two key trends are emerging to address these unmet needs:
- Advancements in formulations, including higher-concentration biologics and the inclusion of recombinant human hyaluronidase (rHuPH20) as a dispersion and permeation enhancer to improve the tolerability of large-volume SC injections
- Innovative device technologies enabling larger volumes and higher concentrations for SC delivery.1
Advancements in drug delivery system technologies have pushed the boundaries of commercial delivery devices, such as autoinjectors and on-body injectors (OBIs), toward higher doses of biotherapeutics through increased volumes and/or drug concentrations, with or without rHuPH20. While significant strides have been made in developing devices for moderate-volume (up to 20 mL) SC injections, challenges persist in addressing the growing need for ultra-large-volume (ULV) SC delivery devices, particularly for volumes exceeding 25 mL. This ULV range includes devices such as OBIs and ambulatory syringe pumps with deliverable volumes between 20 and 60 mL per use, considering drug concentration, wearability and portability.
COMMERCIAL CASES AND CHALLENGES
An important trend to understand is the transition from intravenous (IV) to SC administration. In 2010, the first immune globulin infusion (20%) HIZENTRA from CSL Behring was approved with a syringe pump for delivery volumes of up to 100 mL. In 2019 and 2020, Genentech’s Herceptin HYLECTA® (Trastuzumab/hyaluronidase 5 mL) and PHESGO® (Pertuzumab/trastuzumab/hyaluronidase 10 mL and 15 mL) were approved for manual syringe injections. These successful IV to SC transitions align with the healthcare system’s needs for reductions in resource use, time and cost, along with patient preferences. However, manual syringe injections still require healthcare provider assistance and are typically administered in clinical settings. Moreover, manual injections lasting several minutes can be challenging for healthcare providers and painful for patients.
Currently, the commercial market primarily features autoinjectors with capacities up to 2.25 mL due to short injection duration requirements. Using these devices, higher doses of biotherapeutics can only be achieved by leveraging higher drug concentrations with smaller delivery volumes. Although there are ongoing efforts to develop larger-volume (5–10 mL) autoinjectors from companies such as SHL (Zug, Switzerland), Ypsomed (Burgdorf, Switzerland), Kaleo (Richmond, VA, US) and SMC (Somerset, WI, US), it is widely understood that the advancement of large-volume autoinjector development is reaching its limits in terms of delivery volume and injection duration (approx 30 sec), even with rHuPH20.2–4
“While numerous OBIs are under development, ranging from 5 mL to 20 mL and leveraging conventional drive mechanisms, fundamental challenges remain for ULV applications with 20–60 mL delivery volumes.”
On the commercial OBI combination product side, devices such as West Pharmaceutical’s (Exton, PA, US) 10 mL SmartDose OBI for SC Pharmaceuticals’ Furoscix® (furosemide) and Enable Injections’ (Cincinnati, OH, US) 20 mL EnFuse OBI for Apellis’ Empaveli® (pegcetacoplan) represent the most recent advancements in higher dose delivery. While numerous OBIs are under development, ranging from 5 mL to 20 mL and leveraging conventional drive mechanisms, such as mechanical springs, electromechanical motors, pressure-based systems or elastomeric material, fundamental challenges remain for ULV applications with 20–60 mL delivery volumes. These challenges, based on different drive mechanisms, include:
- Insufficient and inconsistent force/pressure (delivery rate) over the entire injection duration:
• Mechanical drive mechanisms, particularly those relying on springs and elastomeric materials, are susceptible to this challenge due to their inherent strong-to-weak driving force profile. This can lead to issues for drugs that require precise injection duration control and is exacerbated with ULV dose delivery as backpressure increases over time.
• Larger containers require significantly higher driving forces that may exceed the capabilities of springs, elastomeric materials or motors, limiting the selection of standard containers. - Bulky size due to standard container availability and drive mechanism design constraints:
• Standard containers are not space-efficient for wearable and portable device design, especially for delivery volumes exceeding 25 mL. The space around a standard cylindrical container creates unavoidable dead volume for the overall injector size. The addition of a plunger rod further increases the overall device length for syringe pump applications.
• Motor-based drive mechanisms require additional components, such as a gearing system, to handle motion and force transition, increasing the size and weight of the device. - Lack of scalability:
• A platform approach is crucial for both drug and device companies to optimise product lifecycle management, design verification and validation, commercial manufacturing, regulatory submission strategy and complaint handling. Most conventional drive mechanisms lack the scalability to cover a 10–60 mL delivery volume range. - Energy consumption:
• Motor-based drive mechanisms require significant energy consumption to handle the larger driving forces required due to the container size, especially for repeatable injections. - Lack of reusability for mechanical drive mechanisms:
• Mechanical systems, such as springs and elastomeric material, cannot offer reusability, making them less sustainable, due to their inherent limitations on design for recharging the energy required for mechanical OBI applications.
Despite these challenges, OBIs offer more flexibility in terms of drug concentration, injection volume and duration. Considering wearability (size and weight limitations), delivery volumes of up to 60 mL are feasible in an ideal OBI design. This means that the goal of high-dose biotherapeutic delivery with slow and steady delivery rates (long injection durations) and up to 60 mL delivery volumes is achievable. This was further demonstrated through a clinical study by Eli Lilly, which showed the feasibility and tolerability of a low-pain formulation (without rHuPH20) for an abdominal injection of 25 mL at a delivery rate of 0.5 mL/min in both lean and non-lean subjects.5
“Considering the current drug delivery system market and the underlying challenges, there is a clear demand for ULV injectors to address the growing need for high-dose therapies while prioritising patient comfort and convenience.”
Considering the current drug delivery system market and the underlying challenges, there is a clear demand for ULV injectors to address the growing need for high-dose therapies while prioritising patient comfort and convenience.
Considering key factors, from drug formulation to device design and patient preferences, it is essential to focus on developing innovative solutions that can meet the unmet requirements of a space-efficient ULV OBI and syringe pump device that possesses the following characteristics:
- A compact and scalable design in terms of wearability, portability and a platform approach
- A powerful, uniform, controllable and reusable driving mechanism
- Low energy consumption
- Low manufacturing cost.
MicroMED has developed two novel approaches based on a micro-electromechanical system (MEMS)-enabled gas-pressure-driven actuator that possesses these qualities to address the unmet needs in ULV injector devices:
- Dual-bag wearable injector for OBI and ambulatory pump applications
- Gas-bellows-actuated compact/portable syringe pump for ambulatory pump applications.
“The MEMS-enabled gas micropump offers a simple, compact, flexible, robust and powerful energy source to address emerging unmet needs in ULV SC drug delivery systems.”
WORKING PRINCIPLES OF THE MEMS-ENABLED GAS MICROPUMP
MEMS technology enables the integration of mechanical and electrical components on a microscopic scale, allowing for miniaturised high-performance systems. The MEMS-enabled gas micropump offers a simple, compact, flexible, robust and powerful energy source to address emerging unmet needs in ULV SC drug delivery systems.
With its simplicity and low component count for integrating the actuator into the final delivery device, the gas micropump grants the flexibility to adjust the device’s form factor based on the requirements of the final products. The gas micropump employs MEMS technology and electrochemical principles to convert electrolytes into gas pressure in a fast and controlled manner. Inside the micropump, the MEMS microelectrodes, controlled by a printed circuit board assembly, interact with the electrolytes to initiate instantaneous gas generation. This precise control over gas generation enables accurate gas pressure output from the gas micropump.1
The gas pump can be configured as a non-linear actuator, where the actuation is directly performed by the gas micropump system. This flexibility allows the system to adapt to different drug delivery system designs and requirements, such as OBI and ambulatory pump applications.
dBOBi DUAL-BAG OBI – A NOVEL SOLUTION FOR ULV WEARABLE OBIs
MicroMED has developed the dBOBi™, a compact, wearable OBI using dual-bag actuation. Powered by patented MEMS-enabled gas pump technology, the dBOBi OBI surpasses traditional drive mechanism technologies, enabling the SC administration of UVL biotherapeutics. The compact dBOBi OBI device can also replace the existing devices used for ambulatory pump applications.
Key Characteristics
- Dual-Bag Actuation Approach:
As illustrated in Figure 1, two flexible bags (similar to IV bags except for the shape) are constrained inside a half-dome-shaped shell. The MEMS-enabled pneumatic pump pumps gas into the first bag, transferring the pressure to the second, drug-filled bag to initiate the delivery process. The drug can be manually transferred from a vial or syringe through a vial adaptor or a Luer-lock connector to the bag. - Space-Efficient and Lightweight Design:
With very few components inside the half-dome shell, this design enables ULV capacity and scalability for a platform approach to device design. The half-dome design maximises the use of space, enabling delivery volumes that other OBI designs struggle to achieve. - Controlled Pressure Output: This ensures that the target injection duration is achieved and compensates for the higher SC backpressure associated with ULV applications.
- Simple Modular Design: This keeps the cost of goods low and makes manufacturing easier.
- Sustainability: This system can accommodate a design with a separate reusable power module and a disposable drug bag and fluid path system to minimise waste.
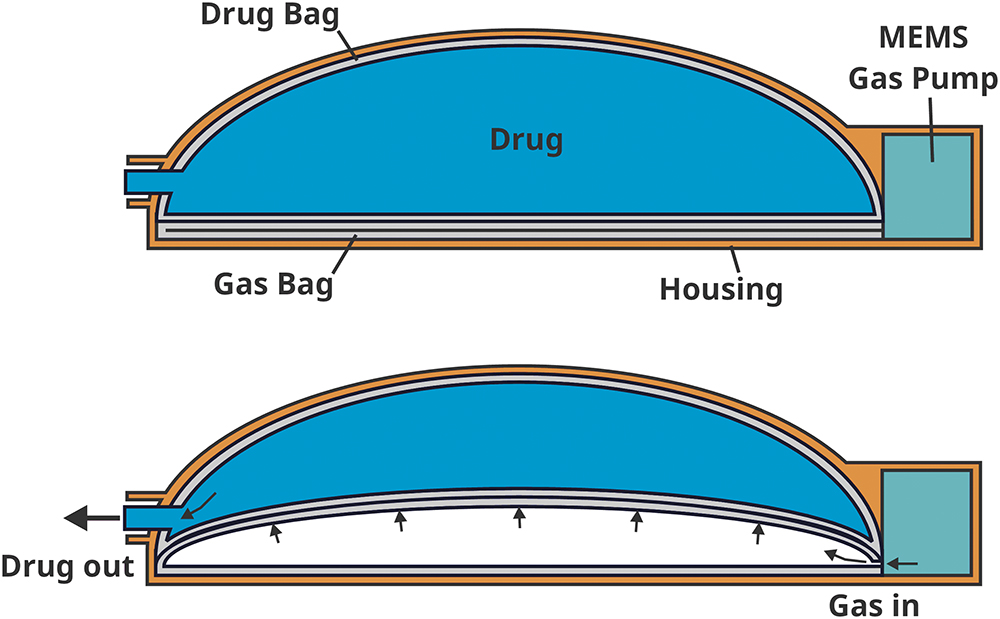
Figure 1: Dual-bag OBI schematics with working principles.

Figure 2: dBOBi dual-bag OBI functional prototype for 50 mL delivery (dimensions: 100 x 90 x 25 mm).
Key Components
Figure 2 shows a functional prototype of a 50 mL dBOBi dual-bag OBI. The size of the 60 mL OBI is comparable to a typical gaming mouse with an even lower height of 25 mm (1 inch).
- Two flexible bags (half-dome shaped) – one for gas, one for liquid (drug)
- One rigid shell (half-dome shaped)
- Battery-powered MEMS gas micropump integrated with the rigid shell and flexible bag for the OBI
- Liquid-transfer connector for transferring the liquid drug from a vial or prefilled syringe into the device
- Needle insertion unit that connects to the drug bag outlet through the OBI.
Key Functions and Operating Steps
- Drug can be manually transferred from a vial or prefilled syringe to the drug bag through the connector
- MEMS gas micropump starts to pump gas into gas bag (volume expansion)
- The volume expansion in gas bag starts (injection starts), squeezing the liquid bag against the rigid shell until the liquid bag is completely empty (injection completes)
- The flow rate is controlled by the MEMS gas micropump
- The liquid bag can be disposed of after use
- The MEMS engine and rigid shell can be reused.
Key Advantages
- Low cost and easy to manufacture due to modular design with minimal components
- Space efficient and lightweight
- Scalable platform approach able to handle doses from 10 to 60 mL
- No risk of drug contamination with dual-bag design separating gas and drug
- Reusable power module with frame
- Replaceable/disposable drug bag with fluid path system
- Low energy consumption – 300 mW (3V, 100mA), 95 mAh per injection of 50 mL deionised water with a 26G thin-wall (TW) needle at 1 mL/min flow rate.
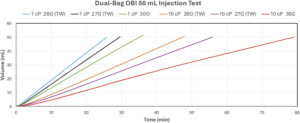
Figure 3: Dual-bag OBI preliminary study results – volume versus time with three different needle gauges and two viscosities.
dBOBi DUAL-BAG ULV OBI – PERFORMANCE AND DISCUSSION
Figure 3 and Table 1 present the delivery volume and delivery duration of the dual-bag OBI prototype using 26G (TW), 27G (TW) and 30G needles with a 50 mL bag of deionised water (1cP) and 60% glycerine solution (10 cP). With alow power input, flow rates ranged from 0.63 mL/min (79 minutes with 10 cP solution and a 30G needle) to 1.94 mL/min (26 minutes with 1 cP solution and 26G (TW) needle) respectively. As shown in Figure 4, the flow rates remained consistent throughout the injection duration for both 1 cP deionised water and 10 cP solutions using 27G (TW) needle.
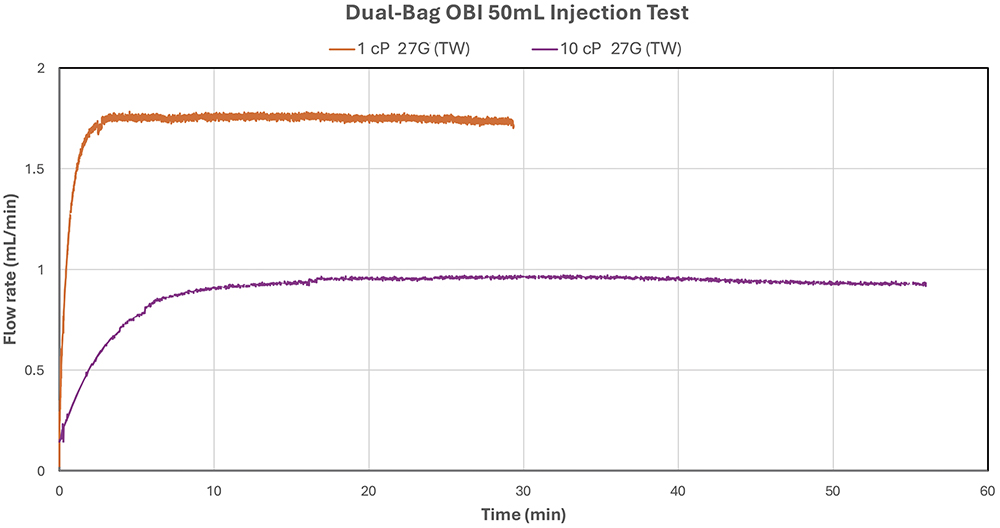
Figure 4: Dual-bag OBI – consistent flow rate over time delivering 50 mL deionised water and 10 cP solution with 27G (TW) needle.
| Test # | Needle | Solution | Viscosity (cP) |
Injection volume (mL) |
Injection time (min) |
Average Flow rate (mL/min) |
| 1 | 26G (TW) | DI water | 1 | 50.2 | 25.9 | 1.94 |
| 2 | 27G (TW) | DI water | 1 | 50.4 | 29.6 | 1.70 |
| 3 | 30G | DI water | 1 | 51.6 | 36.3 | 1.42 |
| 4 | 26G (TW) | 60 % glycerine | 10 | 50.5 | 50.4 | 1 |
| 5 | 27G (TW) | 60 % glycerine | 10 | 50.1 | 55.9 | 0.90 |
| 6 | 30G | 60 % glycerine | 10 | 50.0 | 79.0 | 0.63 |
Table 1: Dual-bag OBI – test results of 50mL delivery of solutions (1 & 10 cP) based on three different needle gauges.
Figure 5 illustrates the results of multiple 50 mL deionised water injections at a target flow rate of 2 mL/min using the 26G (TW) needle. This test demonstrated 24 successful deliveries leading up to the publication deadline. Continued development of the gas micropump is expected to yield a significantly higher number of repeatable injections. These results demonstrate the micropump’s reusable nature and consistent flow-rate performance, fulfilling sustainability requirements.
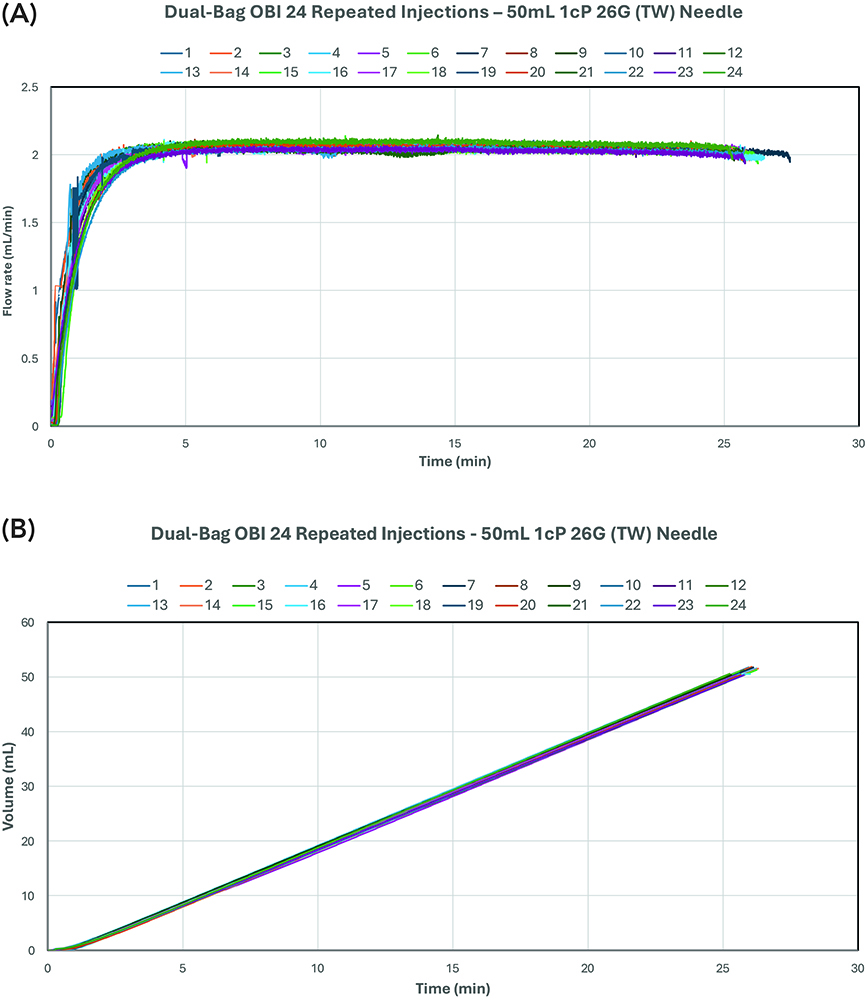
Figure 5: Dual-bag OBI – consistent repeated injections for delivering 50 mL deionised water with 26G (TW) needle: (a) flow rate over time and (b) delivery volume over time.Figure 5: Dual-bag OBI – consistent repeated injections for delivering 50 mL deionised water with 26G (TW) needle: (a) flow rate over time and (b) delivery volume over time.
GAS-BELLOWS SYRINGE PUMP – A NOVEL SOLUTION FOR ULV AMBULATORY SYRINGE PUMPS
The MEMS-enabled gas micropump can efficiently drive a compact, portable syringe pump capable of delivering ULV SC injections with drug volumes over 60 mL.
Key Characteristics
- Gas-Bellows Actuation Approach: The MEMS-enabled pneumatic gas micropump delivers gas pressure directly to the plunger stopper through an extendable bellows, eliminating the need for a plunger rod.
- Reduced Device Size: The gas-bellows device size can be significantly reduced in comparison and become more portable by leveraging gas pressure through bellows to directly move the plunger stopper in the syringe without a plunger rod.
- Constant Pressure Output: This compensates for the higher back-pressure associated with ULV applications.
- High Compatibility: The device can connect with commercially available infusion sets and flow rate controllers.
- Reduced Cost: The gas-bellows approach reduces costs due to its simple design, easy manufacturability, low component count and compact size.
“By eliminating spring-related components and the syringe plunger rod, a 60 mL syringe pump based on a gas-bellows design can achieve a size reduction of approximately 40% compared with existing spring-driven syringe pumps for SC immunoglobin applications.”
Current commercial portable ambulatory syringe pumps for SC immunoglobin are bulky, with a very long length of approximately 25.4 cm (10 inches). By eliminating spring-related components and the syringe plunger rod, a 60 mL syringe pump based on a gas-bellows design can achieve a size reduction of approximately 40% compared with existing spring-driven syringe pumps for SC immunoglobin applications.
Key Components
- One 60 mL disposable Luer-lock syringe with a removable plunger rod
- Gas bellows connecting the plunger/ stopper of the syringe and the gas micropump outlet
- Rechargeable battery-powered MEMS gas micropump integrated with an outer shell accommodating the gas bellows and syringe.
Key Advantages
- Low cost
- Patient comfort with consistent delivery rate
- Compact, portable and lightweight
- Ability to handle 10 to over 60 mL
- Reusable gas-bellows pump
- Replaceable/disposable syringe
- Low energy consumption.
Future Directions and Work
MicroMED has embarked on the development of a gas-bellows syringe pump, integrating a MEMS-enabled gas micropump into a compact, efficient design. This innovative approach uses indirect gas pressure from the micropump, channelled through an expandable gas bellows, to actuate a syringe plunger. Precise control of the gas pressure allows the micropump to achieve accurate and adjustable injection rates. Eliminating the plunger rod significantly reduces the overall length of the gas-bellows syringe pump system, resulting in a low-cost, portable and reusable device. Functional prototypes based on MicroMED’s initial design concepts are currently under development, and the company plans to present its design and preliminary findings in a forthcoming issue of ONdrugDelivery.
“These innovative approaches offer novel and viable solutions for ULV SC drug delivery, particularly in applications such as OBIs and ambulatory pumps.”
CONCLUSION
This article highlights the current market demand for ULV SC drug delivery systems capable of administering higher-dose medications. The challenges associated with conventional drug delivery systems have been identified and addressed through a MEMS-enabled pneumatic actuation system. These innovative approaches offer novel and viable solutions for ULV SC drug delivery, particularly in applications such as OBIs and ambulatory pumps. By overcoming the limitations of traditional driving technologies, the dual-bag OBI and gas-bellows pump approaches demonstrate the potential to deliver high-dose biologics while addressing patient-centric considerations.
REFERENCES
- Yeh H-J, Lai J-R, Cheng T-C, “Advancing Subcutaneous Delivery of Biologics: A Novel Reusable Mems-Enabled Actuator”. ONdrugDelivery, Issue 156 (Jan 2024), pp 117–126.
- Schneider A et al, “Autoinjectors for large-volume subcutaneous drug delivery: a review of current research and future directions”. Expert Opin Drug Deliv, 2023, Vol 20(6), pp 815–830.
- Schneider A et al, “Hold the device against the skin: the impact of injection duration on user’s force for handheld autoinjectors”. Expert Opin Drug Deliv, 2020, Vol 17(2), pp 225–236.
- “Halozyme Announces Positive Clinical Data of its High-Volume Auto-Injector Demonstrating Successful Rapid Subcutaneous Drug Delivery”. Press Release, Halozyme Therapeutics, Oct 2023.5. Dang X et al, “Clinical Investigation of Large Volume Subcutaneous Delivery up to 25 mL for Lean and Non-Lean Subjects”. Pharm Res, 2024, Vol 41(4), pp 751–763.

