Citation: Badelt S, “Critical Lessons Learned: Strategic Planning For Drug Delivery Systems”. ONdrugDelivery, Online, May 19th, 2025.
Dr Steve Badelt of Suttons Creek, a BlueRidge Life Sciences company, discusses how the differing cultures and mindsets common to drug development and device design need to be brought together to achieve a smooth and successful combination product development programme, and explores some best practices for achieving optimal outcomes.
BlueRidge Life Sciences provides comprehensive support for drug and delivery device development across the entire product lifecycle, from nonclinical safety and clinical development to regulatory strategy and commercialisation. Through its extensive experience, the company has seen firsthand that the strategic planning phase is critical to the efficiency and success of a drug delivery system. The most successful development programmes are those that engage expert guidance from the outset, ensuring that strategic decisions, timelines and downstream considerations are carefully aligned to prevent costly setbacks.
With this expertise, BlueRidge has identified the most common industry pitfalls and the strategic practices that drive profitability. Drawing on insights from the combined BlueRidge team of companies, this article highlights key lessons learned to help pharmaceutical organisations – regardless of size or stage – optimise their combination product development and achieve their business objectives.
LESSON 1: KNOW WHEN STRATEGIC PLANNING NEEDS TO HAPPEN
When approaching a combination product development project, it is helpful to look at the work ahead in seven stages to understand the critical steps and prioritisation of resources (Figure 1):
- Strategy
- Quality management system (QMS) setup
- Vendor selection
- Clinical development
- Commercial development
- Regulatory submission
- Launch/post-market surveillance.
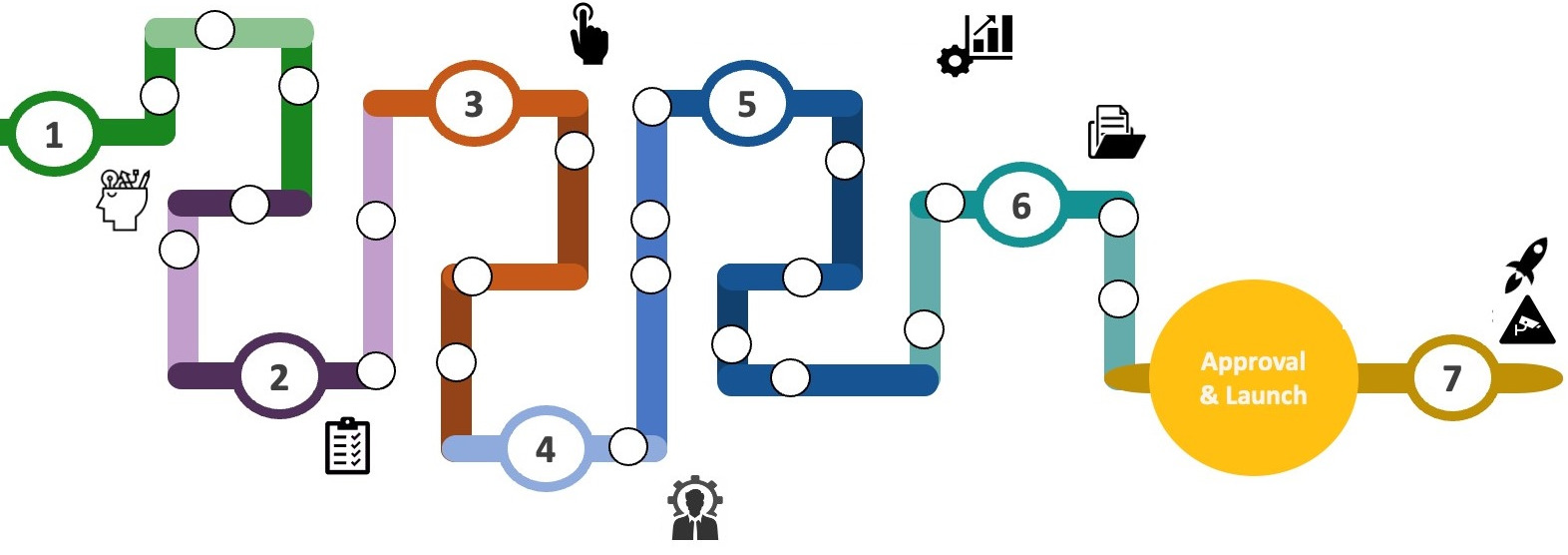
Figure 1: Device development pipeline.
“BLUERIDGE ALWAYS RECOMMEND STARTING AT ‘GROUND ZERO’ WITH A SITUATION AND STRATEGIC OPTIONS ASSESSMENT, BEFORE BEGINNING THE DEVICE STRATEGY STAGE.”
That said, the process of developing a drug delivery system is more complex than this linear view. Drug discovery starts years before any sort of device selection can occur, as the makeup of the drug will mandate certain device design requirements; however, the device that is eventually used for delivery may have constraints that affect drug design requirements. This interdependency creates a more circular, iterative development process. While regulatory submission cannot happen until all of the preceding stages occur; regulatory activities and documentation that support the final combination product submission begin long before the final picture of the drug-device product comes into view.
This is why BlueRidge always recommend starting at “ground zero” with a situation and strategic options assessment, before beginning the device strategy stage. This includes:
- Solidifying the appropriate knowledge base needed to execute the programme
- Understanding key combination product milestones, timelines and costs
- Assessing current QMS and risk management systems (RMS) for best practices and an optimal compliance pathway
- Reviewing potential device and vendor options
- Exploring drug stability and labelled storage conditions
- Assessing human factors requirements
- Understanding any potential interactions between the device materials and the drug product (extractables/leachables)
- Mapping out potential regulatory pathway options, timelines and risks.
When does “ground zero” occur? That will depend on each unique drug product and its intended delivery device, but as a general rule BlueRidge recommends that:
- For combination products using traditional devices, such as prefilled syringes (PFS), autoinjectors, preloaded inhalers and on-body injectors, this assessment begins during Phase II trials as soon as it is time to consider device options and development, regulatory pathway or clinical submission.
- For combination products using novel devices or routes of delivery, such as implantable delivery, microneedle patches, ophthalmic implants and injections or delivery to the central nervous system, this assessment begins during early development or Phase I trials when consideration of a drug delivery device is first discussed.
LESSON 2: A MORE COMPLEX PROCESS REQUIRES A MORE COMPLEX TEAM STRUCTURE AND DYNAMIC
In addition to a more complex development process, combination product development also involves a much more complex team structure (Table 1), including the expected drug team and the device team. It also includes other functional internal team members who are not usually involved in device or combination product development, as well as external device suppliers and vendors who may or may not fully grasp the perspective of the drug side of the equation.
| Device Team/Partners | Drug Team | Other Internal Functions |
| Development/Engineering Device Quality Device Regulatory Human Factors Device OEM Device CMO Device Vendors Project Management |
Drug Product Formulation Supply Chain Manufacturing Quality Control Regulatory CMC Program Management |
Clinical Development Commercial/Marketing Epidemiology Market Access Health Economics (HEOR) Medical Affairs Regulatory (Clinical) Business Development Drug Safety/Pharmacovigilance |
Table 1: Combination product development team structure.
“THIS REQUIRES WORKING WITH A LARGER TEAM MADE UP OF, ESSENTIALLY, ‘DRUG PEOPLE’ AND ‘DEVICE PEOPLE’ WHO NOW NEED TO WORK TOGETHER ON THE COMPANY’S BIGGER PICTURE COMBINATION PRODUCT GOALS.”
This requires working with a larger team made up of, essentially, “drug people” and “device people” who now need to work together on the company’s bigger picture combination product goals. These different backgrounds usually mean approaching the project in two very different manners. First and foremost, their product development processes are different, often as a matter of discovery versus design.
Drug discovery involves identifying new chemical or biological compounds that can treat diseases (Figure 2). This process includes target identification, compound screening, preclinical testing and extensive clinical trials to ensure safety and efficacy before regulatory approval. Characterisation and controls are implemented during clinical development, with cGMPs implemented by phase with controls included and tightened approaching regulatory submission. The drug development process continues to evolve and, more recently, includes elements of intentional drug design (genetically-driven) and “quality by design” approaches to formulation development.
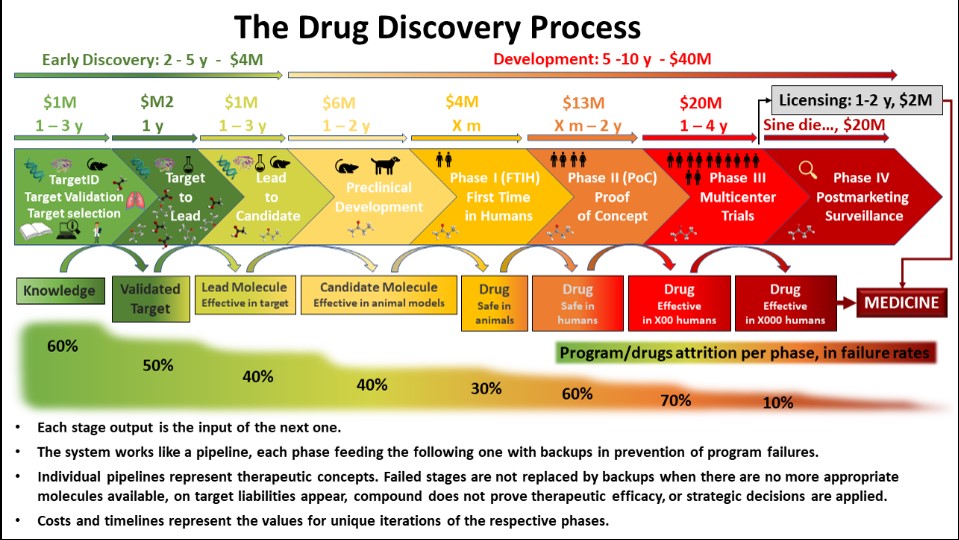
Figure 2: Drug discovery process.
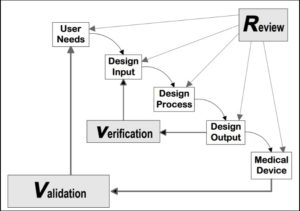
Figure 3: Device design controls.
Devices are designed according to user needs and design requirements for the safe and effective administration of drugs to patients. The design process integrates engineering, materials science, and human factors research to optimise usability, reliability and patient compliance. The process is about engineering optimal therapeutic outcomes rather than discovering new molecules. The device design is documented, then tested (verification and validation), with design controls required from the initiation of development as per cGMP and ISO standards, and any changes to a device between clinical trials and approval must be documented and justified (Figure 3).
Coming from two distinctive approaches means different backgrounds, knowledge, mindsets, priorities, regulatory oversight and terminology for each group. To make working together even more challenging, while a drug plus a device together becomes a combination product, a drug regulatory approach plus a device regulatory approach together is not a sufficient regulatory approach for combination product approval. Combination product regulation is its own unique beast with which many on both sides of the drug-device divide may be unfamiliar. It is important to not just educate the drug and device teams on each other’s processes and goals but to educate everyone on the interdependencies between their two processes and how their processes might need to change specific to combination product approval and launch.
LESSON 3: UNDERSTAND THE IMPORTANCE AND INTRICACIES OF CROSS-FUNCTIONAL COLLABORATION AND ALIGNMENT
Taking a bigger picture view of how device development (usually) fits into the drug development timeline (Figure 4), drug delivery device development typically runs in parallel with drug development but follows a distinct timeline due to the differing regulatory requirements and design considerations. Drug development follows a traditional multi-phase process – discovery and preclinical research (3–6 years), clinical trials (6–7 years) and regulatory review (1–2 years), totalling approximately 10–15 years. Drug delivery device development, on the other hand, follows a medical device development pathway that includes concept development, design verification and validation and regulatory approval, which can take anywhere from 3 to 7 years, depending on complexity and classification.
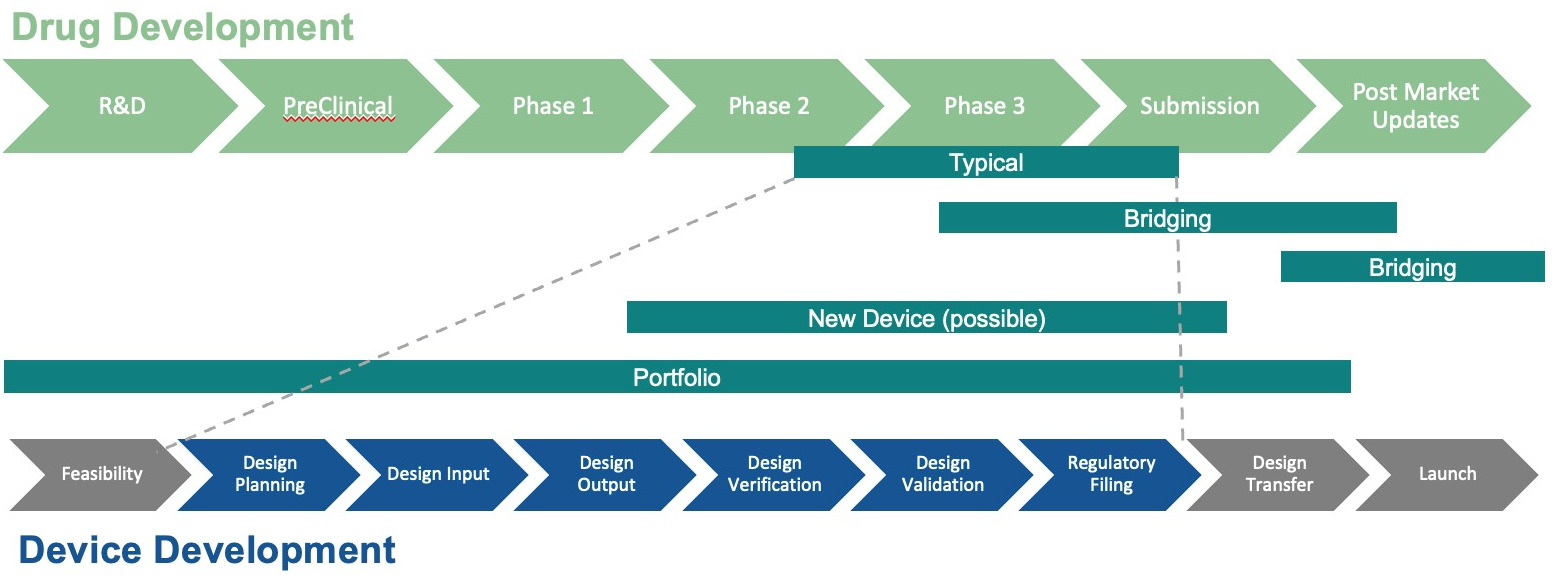
Figure 4: Drug versus device development.
“TO STREAMLINE DEVELOPMENT, BLUERIDGE RECOMMENDS AN INTEGRATED APPROACH WHERE THE DEVICE DESIGN IS CO-DEVELOPED WITH THE DRUG FORMULATION, ENSURING THAT BOTH MEET REGULATORY EXPECTATIONS SIMULTANEOUSLY.”
The timelines overlap most significantly during later clinical phases. During early clinical studies, device prototypes may be tested for compatibility with the drug, ensuring factors such as stability, dose accuracy, sterility, extractables and leachables profile and biocompatibility. By Phase II trials, the device design should be sufficiently mature to support early human testing, though modifications may still be made. By Phase III, the final device configuration must be locked in for regulatory approval alongside the drug. Alignment between these timelines is crucial, as delays in device readiness can slow drug approval, particularly for combination products. To streamline development, BlueRidge recommends an integrated approach where the device design is co-developed with the drug formulation, ensuring that both meet regulatory expectations simultaneously.
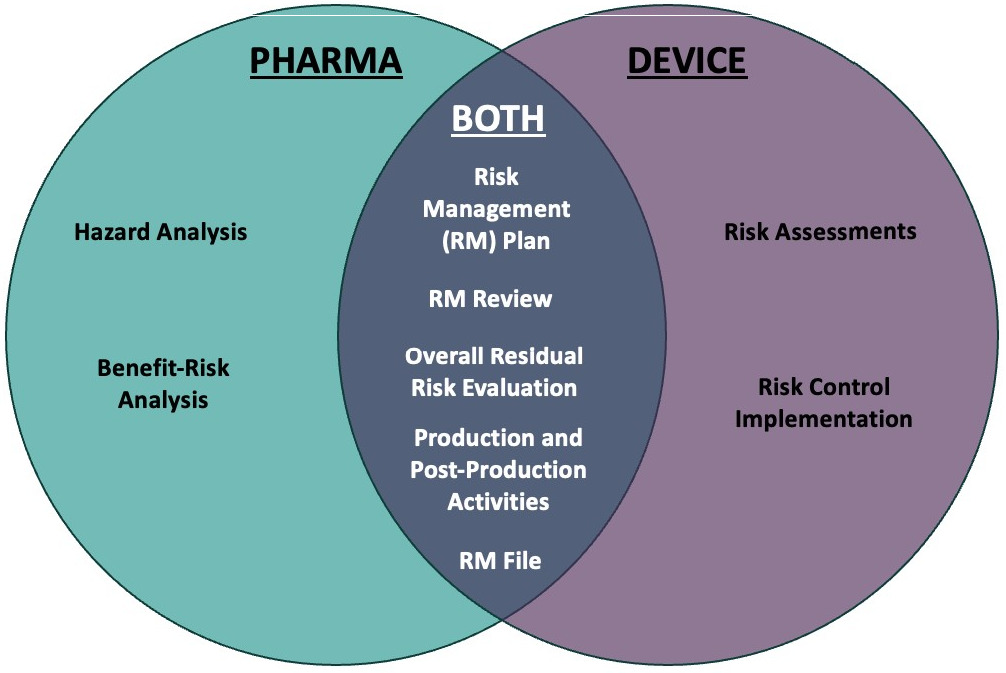
Figure 5: RMS deliverables responsibilities.
Part of getting a cross-functional combination product team working together for co-development is identifying and socialising each function’s responsibilities and cross-functional shared responsibilities across the development and ultimate approval and launch of the combination product. It is critical to align the different approaches to development for a drug and a device to ensure the creation of a complete and efficient regulatory submission. In the example in Figure 5, one can see the collaboration needed between drug and device teams to prepare the needed RMS documentation to include in the combination product’s design history file for regulatory submission.
Another area of critical importance is alignment of QMS. For drug delivery devices and drug products, the QMS is governed by different regulatory frameworks, reflecting their distinct risks, manufacturing processes and performance requirements (Figure 6). A QMS for a drug delivery device will follow medical device regulations, such as ISO 13485 and US FDA 21 CFR Part 820, which emphasise design controls, risk management and device-specific verification and validation. In contrast, a QMS for drug products should align with pharmaceutical regulations, such as FDA 21 CFR Part 210/211 and ICH guidelines, focusing on GMP, chemical stability, sterility and bioavailability. These differences mean that a device-focused QMS will be more concerned with usability, mechanical performance and failure modes, while a drug-focused QMS will ensure formulation consistency, potency and safety.
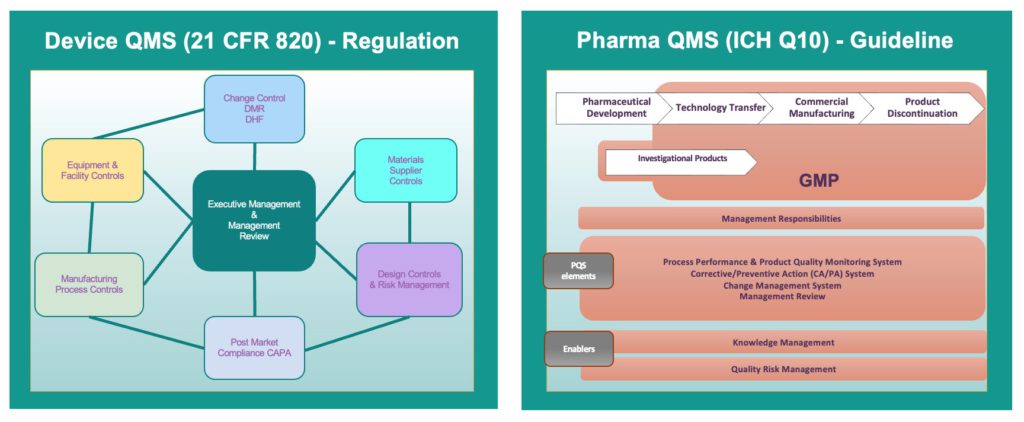
Figure 6: Drug delivery device and drug product QMS are governed by different regulatory frameworks.
For combination product development, aligning these QMS processes is crucial to ensure regulatory compliance and product integrity. A best practice is to implement a harmonised QMS that integrates elements of both systems, leveraging a risk-based approach to manage device-drug interactions effectively. This includes establishing cross-functional collaboration between pharmaceutical and device teams, ensuring that design controls accommodate both drug stability and device performance and integrating risk management per ISO 14971 with pharmaceutical safety assessments. Additionally, clear documentation pathways that satisfy both GMP and medical device QMS requirements can streamline regulatory submissions and lifecycle management. By proactively aligning these systems, companies can enhance efficiency, reduce compliance risks and accelerate market entry for combination products, thereby avoiding costly delays.
All functions involved in product development activities must understand their assigned tasks and their dependencies, or prerequisites, to maximise programme efficiencies and comply with internal development operating procedures and QMS. Dependencies are created for many reasons, such as patient risk reduction, design discovery, minimising development costs risks and avoiding costly mistakes.
In device development, the FDA requires specific tasks be completed prior to another task to minimise risk of design failure; for example, user needs must be completed prior to defining design requirements. In Drug development, a preclinical phase is often required prior to a human clinical trial. In combination product device development, the volume and viscosity of the drug to be delivered must be determined prior to determining the needle gauge. All functions must also understand the impact of certain design and development choices on other functions’ tasks and on the overall timeline and budget. For example:
- Design Choices: The complexity of the drug delivery device and its use define the number of activities and the length of the development timeline.
- Formulation Choices: Decisions on the formulation, concentration, viscosity and volume impact the available design choices.
- Design Changes: Vendor or device changes later in the process increase the number of tests, which mean an increase in the time and money required for development.
“IT IS IMPORTANT TO MENTION THAT THIS ALIGNMENT APPLIES NOT ONLY TO INTERNAL CROSS-FUNCTIONAL TEAMS, BUT CROSS-ORGANISATIONAL TEAMS.”
Finally, it is important to mention that this alignment applies not only to internal cross-functional teams, but cross-organisational teams. Drug delivery device development requires collaboration with external partners – original equipment manufacturers, CDMOs, packaging and labelling companies, testing facilities and more. Each partner has their own systems, processes, communication practices, cultures and expectations for each party’s responsibilities. Getting everyone on the same page for seamless flow of combination product development through each partner’s hands is critical for efficiency and regulatory documentation success.
LESSON 4: IDENTIFY CENTRAL STRATEGIC PLANNING DELIVERABLES
With the foundation built on the previous three lessons, it’s time to dive deeper into “ground zero” and the common questions that need to be answered at the pre-strategy stage, and the strategy stage deliverables that the answers will inform.
Question 1: How Do I Get to Clinical and/or Commercial Launch from Where I Am Today?
- State of the programme discovery and overview
- Milestone and timeline identification
- Development and component cost estimates.
Question 2: What Organisational and Knowledge Capabilities Are Required?
- Functional combination product knowledge gap assessment
- Combination product development alignment gap assessment
- New guidance updates – impact to programme and organisation
- Roles and responsibilities identification and socialisation
- Cross-functional/stakeholder alignment gap assessment.
Question 3: What QMS Do I Need for a Combination Product Programme?
- Existing QMS and product documentation assessment
- QMS compliance pathway options
- RMS options
- Integration pathway with drug QMS
- Integration pathway with vendors’ QMS.
Question 4: What Regulatory Pathway Will Get Me to Launch the Fastest?
- Applicable essential drug delivery outputs and verification/validation requirements
- Device options for clinical application
- Product-specific regulatory pathway options
- Phase-appropriate activities identification
- Cost- and time-saving methods available, such as bridging studies.
Question 5: What Are My Device Options and How Do I Choose?
- Competitive options
- Reliable partnership options
- Vendor management practice review
- RMS needs.
Question 6: What Is the Most Efficient Programme and Systems Setup for a Combination Product?
- Systems engineering best practices
- RMS best practices
- External partner/vendor selection best practices
- Human resources allocation options
- Communications systems best practices.
LESSON 5: BUILD A COMBINATION PRODUCT ECOSYSTEM WITHIN YOUR DRUG WORLD
Even the biggest pharma companies are not necessarily set up for combination product success. The common reason for this is that, as mentioned prior, there is a very different mindset for drug development compared with device development, and the device side is not always understood. Another cause is that drug development, in terms of cost and timeline, dwarfs the resources and attention needed for device development – the drug is the star of the show. However, the delivery device can be a competitive advantage or a market downfall – and can be the reason for launch delays – if it is not given sufficient space and respect in the development programme.

