To Issue 173
Citation: Jørgensen A K, Basit A, Goyanes A, “3D Printing: The Future of Personalised Medicines”. ONdrugDelivery, Issue 173 (May/Jun 2025), pp 22–27.
Anna Kirstine Jørgensen, Dr Abdul Basit along with Dr Alvaro Goyanes, discuss the role of 3D printing in shaping the future of personalised medication, and explain how FABRX’s two pharmaceutical 3D printers can help unlock its potential – extending even beyond Earth.
The “standard medicine for standard patient” paradigm is well-known to be unsuitable, risking the safety of certain patients and patient groups, as well as being a factor for reduced efficacy and treatment adherence.1 Although conventional and large-scale manufacturing processes offer significant cost and production efficiencies, they remain highly inflexible in terms of varying dosage and product characteristics according to patient needs.
Three-dimensional (3D) printing is capable of accurately and effectively producing small batches of pharmaceuticals with tailored characteristics (such as dose differentiation, taste masking and release profiles). This technology is no longer just a crazy vision but is now actively being used to treat patients who are not benefitting from the one-size-fits-all approach.2,3
3D printing is a process whereby a 3D object is designed through computer-aided design software for subsequent printing in a layer-by-layer manner. While upscaling of binder jetting (a specific 3D printing technology) manufacturing processes has resulted in the successful commercialisation of Aprecia Pharmaceuticals’ (Mason, OH, US) SPRITAM® (levetiracetam) to treat epileptic seizures, it remains the only mass-manufactured 3D-printed medication available off-the-shelf to date, nearly a decade after receiving market authorisation.4
Since then, it has become increasingly evident that the small-scale and flexible nature of 3D printing might actually be its major selling point and the key to achieving personalised oral medicines through the so-called “virtuous cycle” (Figure 1).
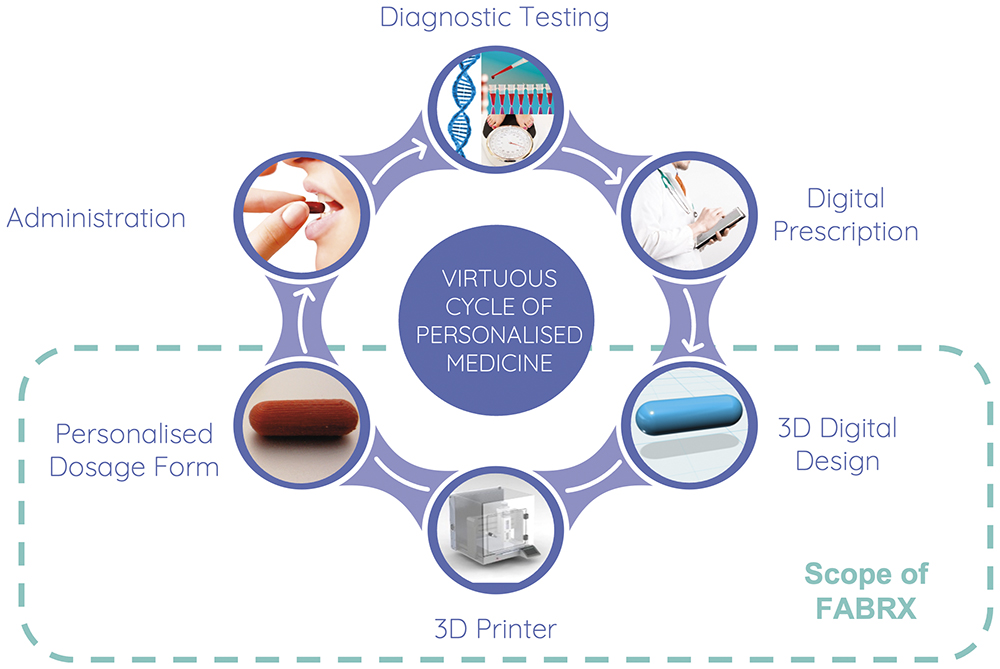
Figure 1: The virtuous cycle of personalised medicines and how FABRX’s 3D printing technologies help realise this.
“FABRX HAS BEEN A DRIVING FORCE IN ADVANCING 3D PRINTING OF PERSONALISED DRUG PRODUCTS FROM NOVEL RESEARCH AND TRANSLATING IT INTO REAL-WORLD CLINICAL IMPACT.”
FABRX has been a driving force in advancing 3D printing of personalised drug products from novel research and translating it into real-world clinical impact.
The versatility of 3D printing in terms of creating unique oral solid dosage forms for a range of different diseases and populations has been demonstrated through several publications. In addition, through partnerships with high-profile institutions, FABRX is now proving how its 3D printing systems can help patients achieve better therapy than currently available options. Even NASA wants to 3D print medications in space to provide better therapy during long-haul missions while greatly reducing occupied space aboard its spacecraft.5,6
As a result of tireless discussions and engagement with regulatory agencies, backed by scientific evidence and case studies, a new paradigm for personalised and on-demand medicines manufacture is now set to commence through new legislative framework.
FABRX’S STATE-OF-THE-ART PHARMACEUTICAL 3D PRINTING SYSTEMS AND SERVICES
Two 3D Printing Setups for Enhanced Flexibility
FABRX has successfully commercialised two pharmaceutical 3D printers (Figure 2), varying in their levels of printing complexities to offer flexibility for the users’ needs. Back in 2020, FABRX launched the world’s first pharmaceutical-grade 3D printer, the M3DIMAKER1, capable of printing with fused-deposition modelling (FDM), semisolid extrusion (SSE) and direct powder extrusion (DPE) technologies through interchangeable printheads. The printer is GMP-certified and comes with inbuilt quality control add-ons, such as an integrated analytical balance for monitoring of mass deposit and a near-infrared spectrometer accessory for API quantification for each individual dosage unit.7,8

Figure 2: FABRX’s two pharmaceutical 3D printers, M3DIMAKER1 (left) and M3DIMAKER2 (right).
The more advanced M3DIMAKER2 was launched in 2023 to enable even more process and product versatility through simultaneous operation of three separate printheads using the same technologies as the M3DIMAKER1, namely FDM, DPE and SSE. The quality control features (analytical balance and near-infrared spectrometer) are also available for this printing system, along with a high-efficiency particulate-arresting filter unit to ensure safe working with hazardous APIs and particle-free printing environments.9
Advanced Software with Easy-To-Use Interface
To help clients and clinicians benefit from all the versatile functions of the printing systems, the M3DIMAKER Studio™ software accompanies the printers. The software allows users to easily select printing models (i.e. size and shape), printing parameters and control the printer. Generating pharma-ink (drug-loaded formulation for 3D printing) profiles accelerates printing of previously developed pharma-inks. The software may be used in R&D mode, where all specifications and parameters are changeable according to the client’s workflow. It can also be operated in “clinical” mode, in which developed and stored protocols can be locked and employed for printing according to the saved protocols.
“FABRX OFFERS VERSATILE CONSULTATION SERVICES FOR ITS CLIENTS TO HELP THEM ACHIEVE THEIR GOALS, RANGING FROM BRIEF CONSULTATIONS TO FULL FORMULATION DEVELOPMENT & ANALYSIS PACKAGES.”
Pharma-Ink Development
Developing the right pharma-ink is important to achieve dosage units with the desired properties and requires general knowledge about formulation and the specific API(s) to be printed. FABRX offers versatile consultation services for its clients to help them achieve their goals, ranging from brief consultations to full formulation development and analysis packages.
CLINICAL TRIALS DEMONSTRATE EFFICACY AND ACCEPTABILITY OF PERSONALISED 3D-PRINTED MEDICINES
FABRX has been involved in two published clinical trials to date, where the efficacy and acceptability of chewable medications tailored to children have been demonstrated. The world’s first clinical study evaluating the efficacy and patient acceptability of 3D-printed personalised medicines was conducted in 2018.10 Children affected by maple syrup urine disease were treated with chewable isoleucine printlets (3D-printed tablets) produced by SSE 3D printing in six different colour and flavour combinations and compared with isoleucine medicines compounded via traditional methods.
Following three months of treatment with each medication type, it was clear that the 3D-printed, chewable dosage units achieved narrower isoleucine plasma levels closer to the target level, and that the 3D-printed pharmaceuticals were well-accepted among the patients. A second clinical study investigated the efficacy and acceptability of treating children affected by rare metabolic disorders with chewable printlets containing more than one active and of different taste and colour profiles (Figure 3).11 The printlets, containing personalised levels of amino acids, were produced in a hospital through the M3DIMAKER1, and good control of plasma levels was achieved for all printlets. The printlets were also well-accepted, and participants indicated that future therapy with these colour- and taste-masked medicines could improve adherence and quality of life.
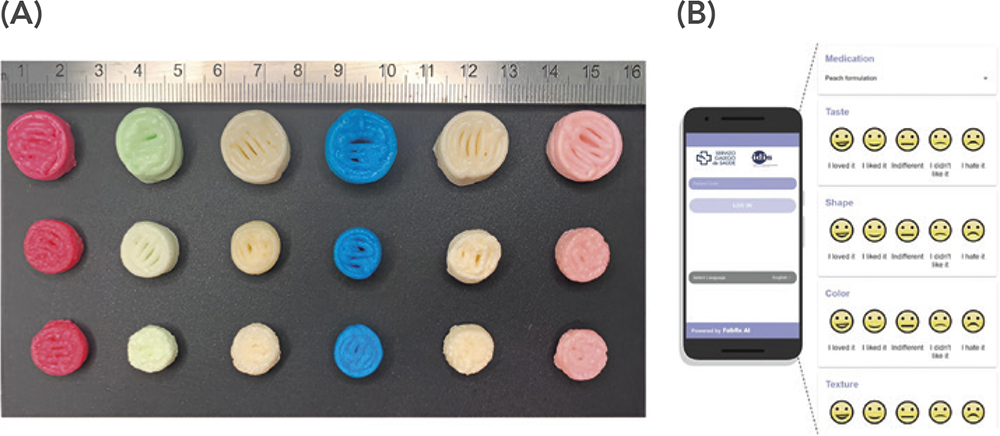
Figure 3: A) Chewable, combination printlets produced in a hospital tested for efficacy and acceptability among children with rare metabolic disorders. Six different colour and flavour combinations were assessed. B) Part of the evaluation process recorded on an app developed by FABRX AI. Figures reprinted from Rodríguez-Pombo L et al. (2024).11
Additional clinical trials assessing the efficacies and acceptability of 3D-printed medicines are already underway. A clinical trial to treat children suffering from adrenal insufficiency with hydrocortisone medicines printed with the M3DIMAKER1 in a hospital is being conducted,12 along with a clinical trial in 200 breast cancer patients treated with multi-active dosage units produced by the M3DIMAKER2 in Europe’s leading cancer research hospital.13
DM OF PERSONALISED MEDICINES IN THE UK
New Legislation Supporting Small-Batch Pharmaceuticals Production
Multiple conversations between developers of 3D-printed personalised medicines and the UK MHRA have now led to new legislation empowering the implementation of decentralised 3D printing manufacturing facilities to produce more bespoke therapies for patients. In the “Statutory Instruments: The Human Medicines (Amendment) (Modular Manufacture and Point of Care) Regulations 2025” legislation signed into law on January 23, 2025, decentralised medicines manufacture may take place through a so-called “modular manufacture” strategy, where multiple decentralised manufacturing (DM) sites will be connected to a licensed centralised control site, responsible for overseeing and ensuring the medicine’s product quality. Parallels can be drawn to “hub-and-spoke” model frameworks, where multiple spokes are connected to a single, centralised hub, which, in the case of modular manufacture, would be represented by modular manufacturing sites connected to a single control site. DM facilities could result in multiple benefits, such as less-sensitive supply chains and the adoption of smaller-scale technologies for more flexible drug production processes.
“THE MODULAR MANUFACTURE FRAMEWORK WOULD ENABLE PRESCRIPTIONS, IN ANONYMISED FORM, TO BE MANUFACTURED AT A DECENTRALISED, MODULAR SITE VIA PHARMACEUTICAL 3D PRINTING.”
The control site is an integral part of the new modular manufacture framework. The MHRA manufacturing licence is given to a control site, which then authorises, on-boards, supervises, oversees, validates, audits and decommissions its modular manufacturing units through a so-called Master File.14 The control site and Master File will be subject to regular inspections by the MHRA, whereas the modular units will undergo occasional inspections to certify appropriate oversight from the control site. Decentralised, or modular, sites manufacturing medications via 3D printing could include licensed pharmacies, both community and hospital, as well as facilities focusing solely on medicines production. With the new legislation coming into effect on July 23, 2025, it is a substantial milestone in moving towards personalised medicines to enhance therapeutic outcomes for patients. While the UK is a first-mover for implementing decentralised manufacture by legislation, other regulatory territories are already in the process of effectuating equivalent frameworks, marking a global shift in the medicines manufacture paradigm.15–17
Envisaged Workflow for 3D Printing Personalised Medicines as Modular Manufacture
The DM sites may produce medicines via GMP-certified 3D printers; hence production of personalised drug products may be attained on much smaller scales than currently possible. In essence, the modular manufacture framework would enable prescriptions, in anonymised form, to be manufactured at a decentralised, modular site via pharmaceutical 3D printing. Product and process data would be obtained through in-line sensors and measures and would be digitally transferred, potentially in real-time, between the modular manufacturing site and the centralised control site, for recording of quality data and quality assurance. Once approved, dispensing of the medicines to the patient may happen either via their point-of-care location (i.e. community pharmacy or hospital) or potentially through delivery directly to their home (Figure 4). Pharma-inks could either be produced directly at the DM sites as part of the modular manufacturing process or in large batches by another site (i.e. a pharmaceutical company) for distribution to the modular sites, as described by a recent case study.8
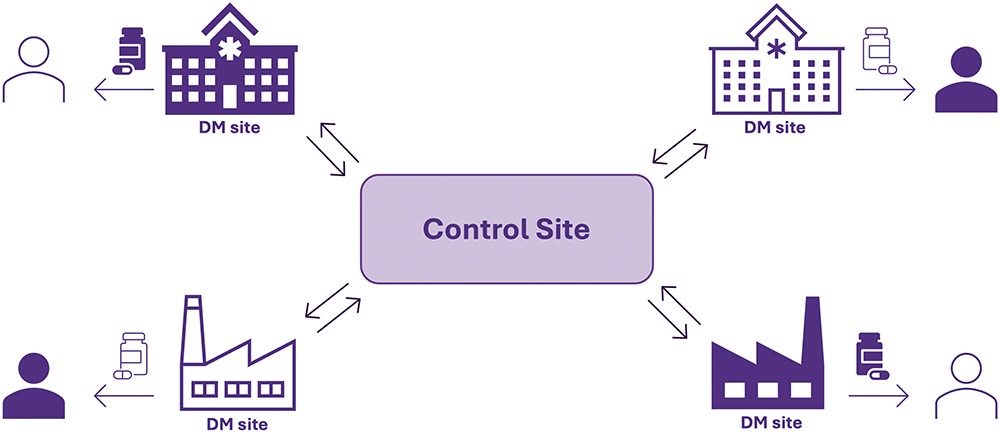
Figure 4: Schematic representation of the new MHRA modular manufacture framework, where multiple DM units are connected to a single control site with two-way communication and data transfer for overall aim of delivering better medicines to patients.
STREAMLINING PHARMACEUTICAL COMPOUNDING – FROM PROCESS TO PRODUCT
Pharmaceutical Compounding – The Original Personalised Medicines
Pharmaceutical compounding is a century-old process where a specific medicine is prescribed for a patient and then prepared by a pharmacist, usually according to specific monographs in national formularies and/or prescriber instructions. The process of pharmaceutical compounding is highly manual, often involving the crushing of commercially available tablets for filling of capsules. Evidently, the process is prone to human-induced errors, which can jeopardise the quality of the final product and thereby patient safety and therapeutic effect. Moreover, compounding requires vast pharmacist resources and is associated with higher costs than off-the-shelf pharmaceuticals. Unsurprisingly, in many countries, pharmaceutical compounding has been heavily reduced even though the practice itself is of great importance for healthcare systems.18 Hence, automating compounding processes, ensuring medications of reproducible qualities, can help to bring much-needed personalised medicines to patients.
“PHARMACEUTICAL 3D PRINTING OF MEDICINES IS ALREADY BEING USED TO TREAT PATIENTS WHERE COMMERCIALLY AVAILABLE PRODUCTS DO NOT MEET THE REQUIREMENTS OF THE SPECIFIC PATIENT OR PATIENT GROUP.”
3D Printing Automates Pharmaceutical Compounding
Pharmaceutical 3D printing of medicines is already being used to treat patients where commercially available products do not meet the requirements of the specific patient or patient group, such as through two M3DIMAKER2 printers in the Clinical Pharmacy department of Gustave Roussy Cancer Campus – a leading cancer research hospital in Paris, France.19 Although 3D printing is considered an “advanced manufacturing technology”, the use of pharmaceutical-grade 3D printers as a means to automate compounding processes is already possible under many existing compounding guidelines. Pharmacists in another European hospital have also been reported to be in favour of automating compounding with 3D printing.20
What pharmaceutical 3D printing offers compared with conventional compounding procedures is greater process control through an automated system, thereby resulting in medicines of greater quality and less room for manual errors. A recent publication has highlighted the benefits of installing a M3DIMAKER2 in a community pharmacy to automate the production and dispensing of oral minoxidil medications directly to patients.21 Correct masses of the medicines were ensured through the integrated analytical balance and an uninterrupted printing process was monitored by the SSE printhead pressure sensor in real-time, all connected to the M3DIMAKER Studio™ software (Figure 5). Overall, the 3D-printed medicines were produced with a 55% reduction in required pharmacist working time compared with conventionally compounded dosage units, thereby providing not only enhanced process and product control but also the potential for resource relocations to bring compounded medicines to a larger array of patients.
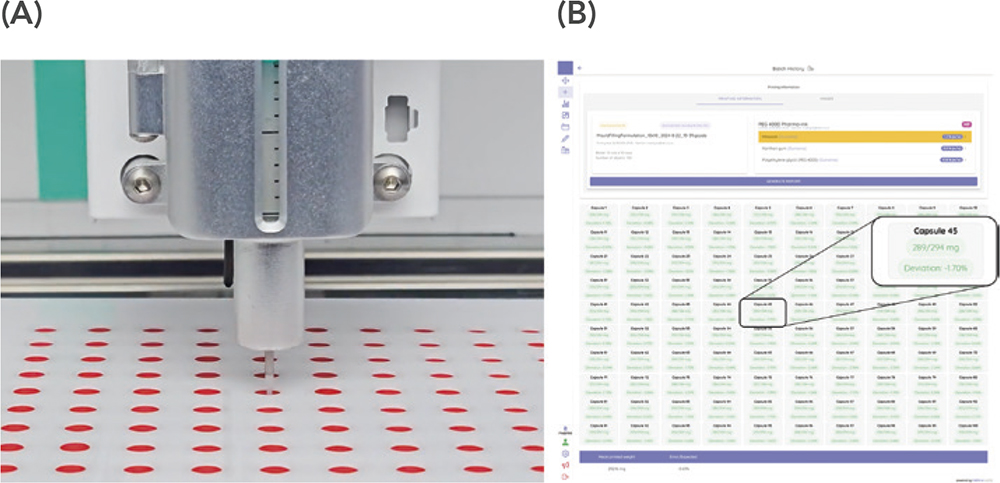
Figure 5: A) Capsule filling via the M3DIMAKER1 printer in a community pharmacy as an automated compounding process resulting in 55% less manual labour. B) M3DIMAKER Studio software interface upon recording of each individual capsule fill weight with indication of mass variation according to target fill weight for individual units and the entire batch. Figures reprinted from Rodríguez-Maciñeiras X et al. (2025).21
CONCLUSION
3D printing of personalised medicines has multiple implications, extending even beyond Earth. Providing more efficient medical therapy during long-duration space missions and space tourism is also on the cards for 3D printing of medicines. Hospital pharmacies are already 3D printing medicines for patients where commercially available products do not meet the patient needs and to conduct clinical trials among these patient groups. Successful implementation of a pharmaceutical 3D printer in a community pharmacy has also highlighted its potential impact for the public by making pharmaceutical compounding more effortless for pharmacists and potentially more accessible overall. Large pharmaceutical companies are currently interested in 3D printing technologies to accelerate prototyping and produce dosage units for first-in-human studies. The future of personalised medicines is being printed – already!
REFERENCES
- “Personalised prescribing: Using pharmacogenomics to improve patient outcomes”. Royal College of Physicians and British Pharmacological Society, 2022.
- Seoane-Viaño I et al, “Translating 3D printed pharmaceuticals: From hype to real-world clinical applications”. Adv Drug Deliv Rev, 2021, Vol 174, pp 553–575.
- Krueger L et al, “Clinical translation of 3D printed pharmaceuticals”. Nat Rev Bioeng, 2024, Vol 2 (10).
- “FDA approves the first 3D printed drug product.” Press release, Aprecia Pharmaceuticals, Aug 3, 2015.
- “3D Printing at University College of London (UCL) School of Pharmacy”. NASA, www.nasa.gov/ochmo-3d-printing-at-ucls-school-of-pharmacy/.
- Seoane-Viaño I et al, “To infinity and beyond: Strategies for fabricating medicines in outer space”. Int J Pharm X, 2022, Vol 4, art 100121.
- Bendicho-Lavilla C et al. “Ensuring the quality of 3D printed medicines: Integrating a balance intoa pharmaceutical printer for in-line uniformity of mass testing”. J Drug Deliv Sci Technol, 2024, Vol 92, art 105337.
- Seoane-Viaño et al. “A case study on decentralized manufacturing of 3D printed medicines”. Int J Pharm X, 2023, Vol 5, art 100184.
- Mora-Castaño G et al, “Optimising 3D printed medications for rare diseases: In-line mass uniformity testing in direct powder extrusion 3D printing!. Int J Pharm, 2025, Vol 668, art 124964.
- Goyanes A et al, “Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients”. Int J Pharm, 2019, Vol 567, art 118497.
- Rodríguez-Pombo L, de Castro-López MJ, et al. Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders. Int J Pharm, 2024, Vol 657, art 124140.
- Rodríguez-Pombo L et al, “3D printed personalized therapies for pediatric patients affected by adrenal insufficiency”. Expert Opin Drug Deliv, 2024, Vol 21(11), pp 1665–1681.
- Denis L et al, “Developing an innovative 3D printing platform for production of personalised medicines in a hospital for the OPERA clinical trial”. Int J Pharm, 2024, Vol 661, art 124306.
- “Collection: Decentralised manufacture hub”. Medicines and Healthcare Products Regulatory Agency, Mar 2025.
- “Distributed Manufacturing of Drugs: Stakeholder Feedback and Action Plan”. US FDA, Nov 2023.
- “Draft Guidance for Industry: Considerations for Complying With 21 CFR 211.110”. US FDA, Jan 2025.
- “Fourth listen-and-learn focus group meeting of the Quality Innovation Group”. European Medicines Agency, Nov 2024.
- Carvalho M, Almeida IF, “The Role of Pharmaceutical Compounding in Promoting Medication Adherence”. Pharmaceuticals (Basel), 2022, Vol 15(9), art 1091.
- “Améliorer les traitements pédiatriques grâce à l’impression 3D.” (“Improving pediatric treatments with 3D printing”.) Press Release, Gustave Roussy, Feb 11, 2025.
- Levine VR et al, “Off-the-shelf medication transformed: Custom-dosed metoprolol tartrate tablets via semisolid extrusion additive manufacturing and the perception of this technique in a hospital context”. Int J Pharm X, 2024, Vol 8, art 100277.
- Rodríguez-Maciñeiras X et al, “Advancing medication compounding: Use of a pharmaceutical 3D printer to auto-fill minoxidil capsules for dispensing to patients in a community pharmacy”. Int J Pharm, 2025, Vol 671, art 125251.

