To Issue 176
Citation: Lee C, Stout M, “Vista By TxSphere: Traversing the Limitations of Small-Volume Biologics”. ONdrugDelivery, Issue 176 (Sep 2025), pp 14–18.
Dr Chaoyoung Lee and Mike Stout unveil an innovative addition to TxSphere‘s wearable platform, Vista, designed to improve on efficiency goals, adapt to varied drug formulations and advance beyond the requirements of low-volume drug delivery.
“THE RESULTING DEVELOPMENT GOAL IS TO BALANCE THE BEST PATIENT EXPERIENCE, TIME TO MARKET, DOSE COST AND SUSTAINABILITY.”
INTRODUCTION
Bringing a biopharmaceutical to market represents an enormous undertaking. Furthermore, the choice of how to deliver the preparation is much more complex than most pharmaceutical products. The push to transition from intravenous (IV) to subcutaneous (SC) delivery, especially for self-administration, adds even more considerable challenges. How best to meet these challenges is a strategic decision that impacts the entire lifecycle of a therapeutic product. The resulting development goal is to balance the best patient experience, time to market, dose cost and sustainability.
THE SHIFT FROM IV TO SC ADMINISTRATION
Historically, the high doses required for many biologic therapies have necessitated IV administration in clinical settings – a method that is costly, time-intensive and inconvenient for patients. In response, the pharmaceutical industry is increasingly shifting towards SC delivery. This transition is fuelled by several compelling drivers – the convenience of at-home self-administration, improved patient adherence and a substantial reduction in the economic burden associated with infusion-based care.
Transitioning to SC delivery presents significant technical challenges, particularly in formulation and device design. SC injections are constrained by limited volume tolerance, meaning that the high doses required for protein-based biopharmaceuticals introduce additional complexities such as increased viscosity and formulation instability.
Formulation Challenges
SC formulations traditionally involve increasing drug concentration to reduce injection volume, enabling rapid administration via autoinjectors or manual injection. These small-volume, highly concentrated formulations present significant technical hurdles not found in low-concentration preparations.1 Elevated protein concentrations lead to increased solution viscosity, which can complicate manufacture and hinder injectability. Additionally, high concentrations elevate the risk of protein aggregation, opalescence and physical instability.2
Another approach involves the use of permeation enhancers, which can dramatically increase the volume of fluid that can be delivered subcutaneously. However, they also contribute to the overall formulation volume, often exceeding the capacity of conventional delivery devices.
Delivery Device Challenges
The autoinjector is a widely used device for the self-administration of biologic drugs. Its design focuses on facilitating simple and rapid drug delivery for the user, enabling self-administration of a therapeutic dose in a single action. The primary engineering consideration in autoinjector development is to deliver a complete dose of viscous drug formulations within a period generally regarded as suitable for patients (usually 15–20 seconds or less).3
This situation creates a complex relationship between device mechanics, formulation properties and clinical pharmacology. To achieve the necessary injection force, manufacturers frequently employ stronger springs, which can place greater stress on the primary container, increase device size and raise the amount of force during injection. This increased stress also necessitates more robust container designs and materials.4 Autoinjectors are also constrained by volume limitations, with most commercially available devices supporting doses in the 0.3–2.25 mL range, with some emerging devices expanding this up to 5 mL.
While some of these formulation and delivery challenges can be mitigated, further increases in concentration and reductions in dose volume are ultimately constrained by the physical limits of formulation and the capabilities of current delivery technologies. As viscosity, stability and device performance thresholds are approached, trade-offs become increasingly difficult to manage without compromising safety, efficacy or patient usability.
“AN ALTERNATIVE TO FORMULATING OR DELIVERING BIOLOGICS AT HIGH CONCENTRATIONS IS TO PLACE LESS EMPHASIS ON SMALL VOLUMES AND MORE ON OPTIMAL FORMULATIONS.”
THE LARGE-VOLUME ALTERNATIVE
An alternative to formulating or delivering biologics at high concentrations is to place less emphasis on small volumes and more on optimal formulations. This approach offers numerous benefits.
Transitioning to large-volume protein formulations removes the limitations associated with small-volume delivery. This strategy has far-reaching implications for the drug development process, including:
- Viscosity Reduction Benefits: This can ease manufacturing processes, enhance formulation stability and reduce the need for high-force delivery mechanisms.
- Accelerating Development: Large-volume alternatives help to reduce the necessity to re-formulate, potentially shortening development timelines and lowering associated costs.
- Enabling High-Dose Biologics: Certain biologic molecules, particularly at high doses, are difficult, or even impossible, to concentrate at levels compatible with conventional self-administration devices.5
Large-Volume Delivery Benefits
Capitalising on the benefits of large-volume formulations will require a different approach to administration. Large-volume on-body delivery systems are expected to be part of the solution.6 Devices that deliver at slower rates can further expand the utility by mitigating discomfort.4,7
Slow infusion allows for gradual absorption and dispersion of fluid through SC tissues and the lymphatic system, minimising discomfort and improving tolerability. When administered at a controlled rate, even relatively large volumes can be delivered comfortably and effectively. A notable example is the transition of immunoglobulin therapies from IV to SC delivery, where volumes of 50–60 mL per injection site are successfully administered over a 60-minute period.
Addressing the challenge of delivering larger volumes via the SC route without compromising patient comfort can be achieved through innovative wearable technologies with adaptable delivery profiles. Large-volume wearable devices embody this shift in philosophy – moving away from the rapid, forceful injections of autoinjectors and prefilled syringes towards a more patient-friendly approach that emphasises comfort, tolerability and extended wear. By prioritising volume capacity over injection speed, these devices unlock new possibilities for SC administration, including the delivery of biologics and other complex therapies.
“TXSPHERE’S PHILOSOPHY IS THAT DEVICES SHOULD ADAPT TO THE DRUG FORMULATION, NOT VICE VERSA.”
TXSPHERE’S MODERN APPROACH TO WEARABLE DESIGN
Higher dose volumes pose design challenges for wearable devices as well. Extended wear times require careful consideration of patient acceptance, such as the clarity of its user interface, size and comfort. For the pharmaceutical company, it is also important for delivery devices to be adaptable to various dosing regimens. TxSphere develops wearable devices capable of administering biopharmaceuticals across a range of dose volumes and concentrations. This adaptability can enable pharmaceutical manufacturers to reduce the amount of time spent adjusting formulations to suit various delivery devices. In other words, TxSphere’s philosophy is that devices should adapt to the drug formulation, not vice versa.
Last year, TxSphere launched Horizon III, a small wearable device capable of delivering at least 10–20 mL of medication while not much larger than an insulin patch pump (Figure 1).
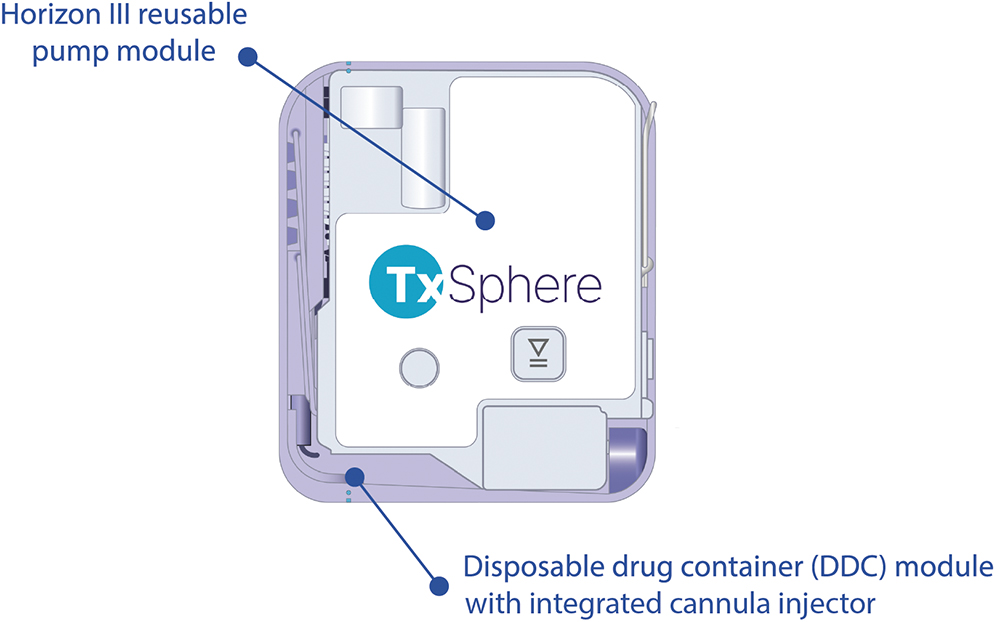
Figure 1: The Horizon III Wearable.
This small size was made possible through miniaturisation of TxSphere’s fluid delivery technology and an internal reservoir. The internal reservoir of the Horizon III has the advantage of reducing the overall size of the wearable and does not require stoppers, movable plungers or lubricants.
Nevertheless, this approach involves a trade-off in terms of reduced pre-filling efficiency. Filling at the point of care is another suitable option, however it introduces additional complexity for self-administration.

Figure 2: The new Vista wearable (10 mL configuration).
THE NEW VISTA WEARABLE – STANDARD PRIMARY PACKAGING
Out of its commitment to tailoring our devices to the requirements of the drug product, TxSphere is pleased to announce the addition of Vista to its wearable platform (Figure 2). Vista retains much of the innovations of Horizon III, but instead of an internal reservoir, Vista delivers medication from standard vials or cartridges. The addition of primary packaging for Vista required a moderate increase in size, but it still maintains the compact, patient-friendly form factor and other benefits of Horizon III.
With the addition of Vista, TxSphere now offers two versatile wearable designs, each providing a discrete combination of benefits:
- Horizon III is designed to prioritise the patient experience while offering a less efficient filling process
- Vista is primarily focused on greater efficiency in filling, storage and distribution processes.
The TxSphere Wearable Platform
Both the Vista and Horizon III wearables are built on TxSphere’s two-piece modular platform, which consists of a reusable pump and a disposable drug container (DDC).
The pump module is based on TxSphere’s core technology – a linear volumetric peristaltic pump, which is derived from the company’s extensive experience in infusion pump development. The disposable module contains the drug reservoir and an automated soft cannula injector.
The integration of TxSphere’s miniature pump module with the DDC works seamlessly across a range of primary packaging systems. This design enhances the patient experience, supports drug-specific dosing needs – including concentration, volume and viscosity – and addresses critical factors in container filling, storage and distribution throughout the product lifecycle.
Improved Patient Experience
If a device is truly “wearable”, it must meet the practical expectations of wearability – ensuring comfort, discretion and ease of use for the patient throughout the intended wear period.

Figure 3: Vista’s compact size.
- Comfort: The compact and lightweight design means it is less noticeable and easier to conceal. The automated cannula injector is hidden from the patient and provides extended wear comfort when compared with a needle. Cannula insertion is quick and much gentler than typical autoinjectors (Figure 3).
- Simple Operation: Requires just a few steps – insert the vial into the DDC, connect the pump, adhere it to the skin and press Run. Sensors verify each action before medication delivery, reducing user errors and preventing mistakes that can lead to device incapacitation and wasted doses (Figure 4).
- Hands Free: Does not need to be held in place during delivery, which typically takes around 15 seconds with an autoinjector. This hands-free method allows the patient to carry out regular activities during the infusion.
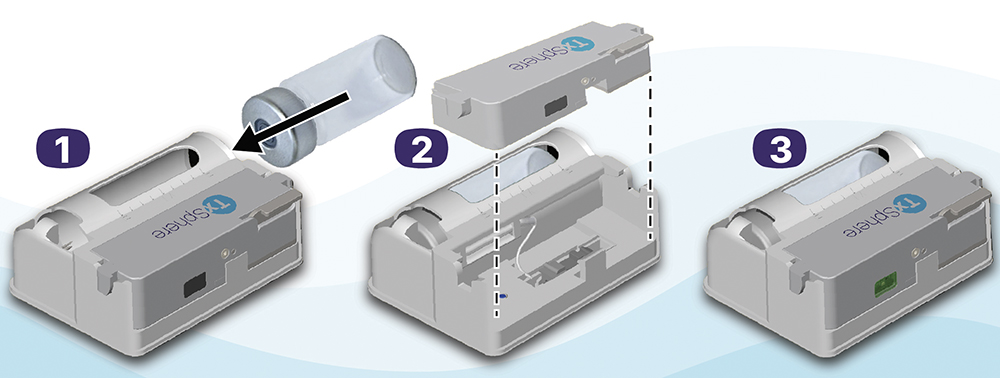
Figure 4: Vista preparation – three steps.
“NEITHER THE MEDICATION NOR CONTAINERS ARE SUBJECT TO HIGH PRESSURES AND THE RELATED VISCOUS FLOW CONCERNS, SUCH AS THE NECESSITY OF SPECIALISED, ROBUST CONTAINER SOLUTIONS.”
Container Simplicity and Flexibility
Because TxSphere wearables pull medication from the drug reservoir, many container challenges are reduced or eliminated. Neither the medication nor containers are subject to high pressures and the related viscous flow concerns, such as the necessity of specialised, robust container solutions.
The two reservoir configurations offer increased flexibility to meet diverse development and manufacturing needs:
- Internal Reservoir (Horizon III): This integrated, prefillable reservoir eliminates the need for stoppers, movable plungers and lubricants – reducing potential sources of variability.
- Standard Primary Packaging (Vista): Traditional vial formats offer proven stability, ease of filling and compatibility with existing infrastructure. This approach can accelerate development timelines by minimising the need for new packaging solutions. Cartridges provide another familiar and scalable option.
“THE VERSATILE DESIGN OF TXSPHERE WEARABLES ACCOMMODATE A WIDE RANGE OF DIFFERENT DOSE VOLUMES, DELIVERY RATES AND VISCOSITIES, ALLOWING DEVELOPERS
TO LEVERAGE THE ADVANTAGES OF LOWER-CONCENTRATION FORMULATIONS.”
Formulation and Dosing Flexibility
The versatile design of TxSphere’s wearable devices accommodate a wide range of different dose volumes, delivery rates and viscosities, enabling developers to leverage the advantages of lower-concentration formulations, including added permeation enhancers:
- Reduced Time and Cost: A more efficient development process can result in substantial cost and time savings.
- Accelerated Time to Market: Less time spent on costly formulations and container challenges, helps to get products to the market faster.
Sustainability
Sustainability has emerged as an increasingly important consideration in recent years. Nevertheless, many wearables and autoinjector devices in the market require the disposal of the entire unit following each use. Vista’s modular design addresses this challenge by enabling the reuse of electromechanical components, with only the sterile fluid path elements being discarded after each dose. This approach also offers the added advantage of reducing the cost per dose.
SUMMARY
The transition from IV to SC delivery for biopharmaceuticals presents new challenges across an already intricate product lifecycle. Navigating these complexities requires strategic decisions that shape every stage of development. Equally important is the selection of a delivery device, which plays a pivotal role in product strategy and should be integrated into lifecycle planning as early as possible.
TxSphere’s wearable platform – Horizon III and Vista – offers a modern alternative to meeting many of these challenges. Supporting a broad range of volumes, formulations and packaging formats, these devices align with the evolving needs of today’s drug-device combination products.
REFERENCES
- Garidel P et al, “High-Concentration Protein Formulations: How High is High?”. Eur J Pharmaceutics and Biopharmaceutics, 2017, Vol 119, pp 353–360.
- Ren S et al, “Current and emerging strategies for subcutaneous delivery of high-concentration and high-dose antibody therapeutics”. J Pharm Sci, 2025, Vol 114(8), art 103877.
- Rini C et al, “Enabling faster subcutaneous delivery of larger volume, high viscosity fluids”. Expert Opin Drug Deliv, 2022, Vol 19(9), pp 1165–1176.
- Badkar A et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- Jansen P, “2014 to 2018: An Update on the State of Wearable Injectors”. ONdrugDelivery, Issue 90 (Sep 2018), pp 6–8.
- Bittner B et al, “Advancing Subcutaneous Dosing Regimens for Biotherapeutics, Clinical Strategies for Expedited Market Access”. BioDrugs, 2024, Vol 38, pp 23–46.
- Akinseye C et al, “Investigation into the Acceptability of Moderate-to-Large Volume Subcutaneous Injections in Healthy Volunteers: Results from a Single-Center Randomized Controlled Study”. Med Devices (Auckl), 2024, Vol 17, pp 369–384.

