To Issue 177
Citation: “Interview with Laxman Halleppanavar: Unlocking the Pent Up Demand for Dual-Chamber Delivery Systems”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 8–12.
>> Press release announcing key partnership between Credence and SMC Ltd, here <<
Q To open, can you tell me what you see are the main industry trends and external environmental factors that are posing challenges to injection delivery systems?
A Starting with some general industry trends, the first ones that come to mind are pharma embracing heightened sustainability goals, patient-centric care and transitioning more treatment away from formal healthcare settings to at-home administration. This is pretty evident these days for many drug categories.
Another important trend stems from the rising use of biologics, including long-acting drugs, which is driving demand for higher delivery volumes and increasing concentration, impacting drug product viscosities. Furthermore, certain therapeutic areas have increasing requirements on higher dose accuracies with low volumes.
Something else you might have noticed is that manufacturing capacity constraints are having an impact at pharma companies’ own sites, especially with glucagon-like peptide-1 receptor agonist (GLP-1) drugs. As a result, we’re seeing significant movement of manufacturing towards CDMOs. These companies are having to expand to meet demand, which is driving higher lead times for equipment manufacturers.
Moving on to some key external factors that are posing challenges to injection delivery systems. The first one that springs to mind is increasing regulatory scrutiny and evolving guidelines – this has been particularly noticeable in the area of combination products over the last few years. Everybody’s learning, including the regulators, so it’s critical to pay attention to upcoming and draft guidelines and how they’re impacting the industry. For example, EU Annex 1 compliance, which is focused primarily on drug filling, is putting pressure on the cost of drugs even as governments are trying to push the cost of medications down, creating both a push and pull simultaneously that the industry needs to navigate. There’s an increased level of supply chain volatility that we’re seeing due to geopolitical pressures, which is creating significant pressure on manufacturing costs.
Alongside that, you’ve got the question of upcoming intellectual property and patent cliffs, which increases the need for pharma companies to demonstrate product differentiation. Based on the current state of the market, this has to be one of the key considerations for pharma companies going forward.
Q These are certainly changing times, presenting challenges but also opportunities and demand for innovative approaches, which leads us to the next question. What are the trends and challenges driving the need for dual-chamber syringe systems specifically?
“WHEN IT COMES TO MORE COMPLEX INJECTABLE THERAPIES, THE MOVE TO AT-HOME SELF-ADMINISTRATION DOES POSE INCREASED RISK TO LESS EXPERIENCED USERS BECAUSE EXISTING VIAL KITS CAN BE COMPLICATED AND UNINTUITIVE TO USE.”
A Well, there’s an overlap with some of the general trends we’ve just discussed. But, focusing on dual chamber specifically, the critical drivers are the move from formal healthcare settings to at-home administration and the increasingly complex formulations being developed. The benefits of this move to the home are myriad and well-documented, both for patients and for healthcare systems. However, when it comes to more complex injectable therapies, the move to at-home self-administration poses increased risk to less experienced users because existing vial kits can be complicated and unintuitive to use, especially with therapies that involve multiple liquids or require reconstituting a lyophilised drug.
That neatly brings me to the other key trend related to dual-chamber devices – an increasing number of therapies are being developed as combinations with each other, leading to liquids that cannot be co-formulated but need to be co-administered. Simultaneously, we’re seeing an uplift in the number of low-stability formulations packaged as lyophilised powders, which then require reconstitution at the point of care. These are both increasingly common practices, which is evident from the volume of vial kits being produced. These factors complicate the injection delivery process and introduce significantly increased opportunities for user error compared with a single injection from a prefilled syringe (PFS) – add to that the increased regulatory pressure demanding that potential dosing errors and contamination be minimised and it becomes clear how a dual-chamber delivery system would offer significant advantages.
Last but not the least, we need to consider pharma’s heightened focus on sustainability goals across the three ESG pillars – environmental, societal and governance – a major factor of which is waste reduction. This means eliminating the waste in terms of overfilling vials or needing to provide multiple vial kits to patients, each of which includes multiple vials, multiple syringes, multiple swabs, etc. Compare that with a single prefilled dual-chamber syringe and it is self-evident which option best aligns with sustainability goals.
Q What are the key features of the Credence Dual Chamber system and its adjacent technology offerings?
A The Credence Dual Chamber Syringe System offers four configurations – liquid-liquid sequential, liquid-liquid reconstitution, lyo-liquid reconstitution and powder-liquid reconstitution (Figure 1). Then, within those four main configurations, there is scope for multiple variations. That’s the core of our technology.
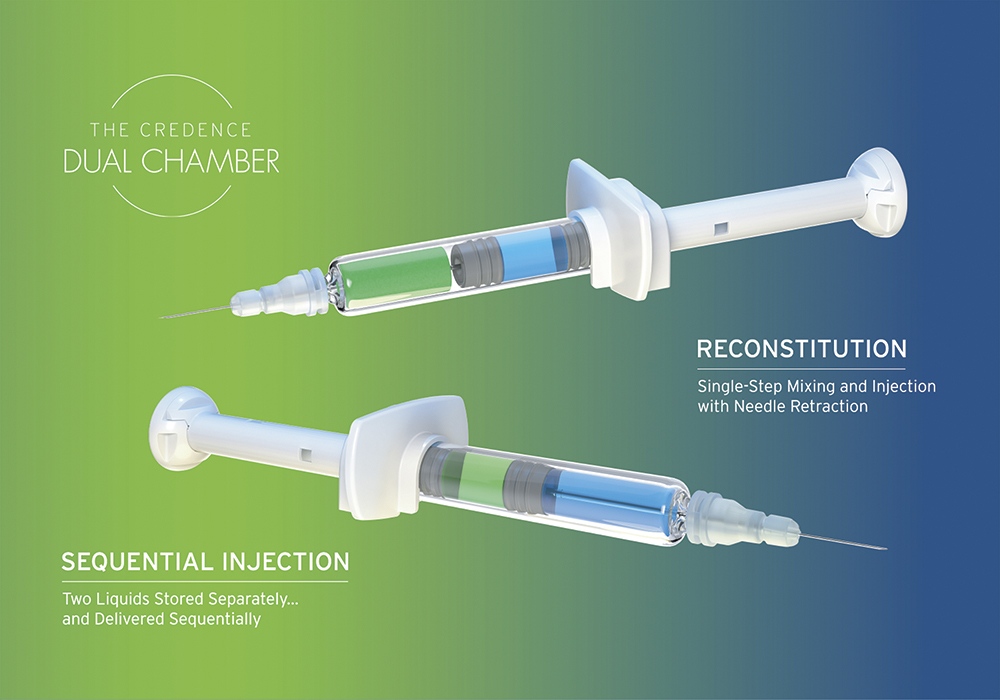
Figure 1: The Credence Dual Chamber System is available in four configurations – liquid-liquid sequential, liquid-liquid reconstitution, lyo-liquid reconstitution and powder-liquid reconstitution.
Building on what we were discussing before, we’ve ensured that the Credence Dual Chamber is very simple to use, as the usability remarkably approaches that of a standard single-chamber PFS. With a simple push down on the plunger, you mix the contents in the syringe itself in a single step, eliminating the complexity inherent to vial kits. You don’t need a lot of training there. Furthermore, there is an audible click at the end of the dose, with a simultaneous tactile sensation, to indicate that the complete dose has been delivered.
“THE CREDENCE DUAL CHAMBER IS OFFERED IN TWO MAIN FORMATS – A RETRACTABLE PASSIVE NEEDLE SAFETY SYSTEM AND A STANDARD LUER LOCK CONNECTION.”
The Credence Dual Chamber uses standard ISO class syringe barrels offered by the leading manufacturers. Building on that, the Credence Dual Chamber is offered in two main formats – a retractable passive needle safety system and a standard Luer lock connection. The system is designed to work with a wide range of volumes, from 1 mL up to 100 mL, or even 200 mL in some extreme applications.
Another important facet to note here comes from the human factors (HF) studies that have been conducted comparing our Sequential Dual Chamber System with a standard single-chamber PFS. When you take a single-chamber PFS, you just push the plunger rod and the drug gets delivered – simple. The same is true of our Sequential Dual Chamber System, except two different liquids are delivered. What we’ve seen in our HF studies is that users have no preference for the single-chamber standard PFS compared with the Credence Sequential Dual Chamber Syringe. That’s extremely significant, demonstrating that in this case, you get two liquids delivered for the ‘price’ of one.
We also have some key adjacent technologies, one of which is focused on giving the user more control of the injection. To address this, we’re developing a Manual Injector, which essentially encapsulates a dual-chamber syringe in ergonomically friendly housing. The result is a system that performs many of the duties of an autoinjector, such as being user-friendly, needle guarding and end-of-dose cues, but maintains the control of the injection with the user and achieves more economical cost targets.

Figure 2: Credence’s Red Dot 2025 award-winning autoinjector, which integrates Credence’s Sequential Dual Chamber System and 3 mL Companion Syringe.
Complementary to that, we are developing a more traditional autoinjector that integrates with our Sequential Dual Chamber System. This is a collaboration with Cambridge Design Partnership, and we are thrilled to say that the design won a Red Dot technology award for 2025 (Figure 2). Having a range of options all built off the Credence Dual Chamber – a PFS, manual injector and autoinjector – gives customers real flexibility in finding the dual-chamber solution that best fits their user needs, all while enabling faster development timelines and reduced cost.
Q What have been the barriers preventing pharma companies from adopting dual-chamber systems?
A One of the major barriers is that historical dual-chamber systems have not had the usability or the inherent safety to allow easy and proper administration of these medicines that require mixing at the time of use. The conventional approaches required a full autoinjector device around the primary package to allow use, and the number and complexity of use steps was still onerous. The devices weren’t ready.
But neither was the infrastructure in the supply chain. Thus far, the industry hasn’t had established “mainstream” dual-chamber fill-finish capabilities available to pharma manufacturers. I think it’s fair to say that the understanding of the dual-chamber opportunity has in the last couple of years really started to gain significant momentum. In order to enable the opportunity for dual-chamber delivery systems to be unlocked, a broader availability of CDMO fill-finish capability is needed (Figure 3).
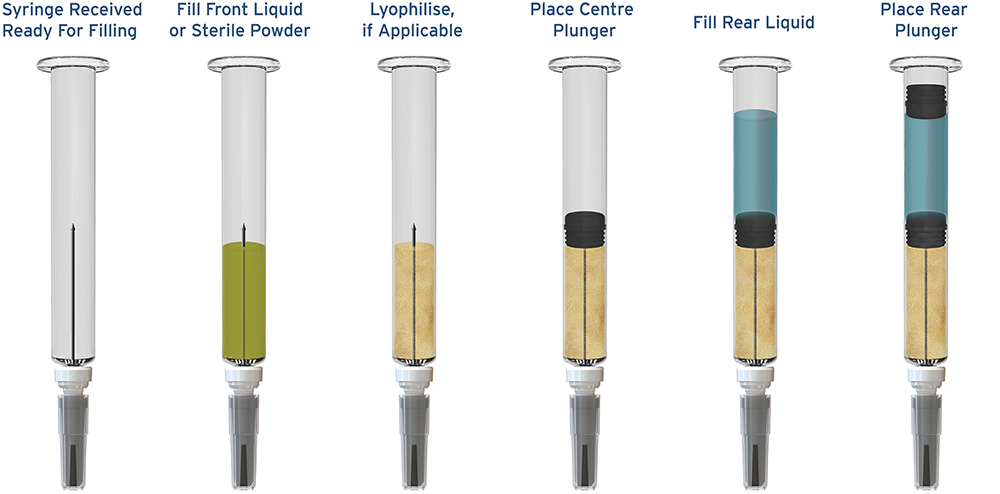
Figure 3: The Credence Dual Chamber Syringe enables broader fill-finish options from CDMO partners.
“FORMULATION SPECIALISTS WITHIN THE PHARMA COMPANIES MAY NOT HAVE FULL EXPOSURE TO WHAT’S OUT THERE – IN MANY CASES THEY MAY NOT KNOW THAT THE IDEAL SOLUTION IS ALREADY AVAILABLE WAITING TO BE ADOPTED, SO IT’S CRITICAL FOR TECHNOLOGY DEVELOPERS TO INCREASE AWARENESS OF WHAT’S AVAILABLE.”
There also may be some unfamiliarity within broader pharma organisations regarding the advancements in drug delivery systems that the industry has been developing, and dual-chamber systems are no exception. Generally speaking, formulation specialists within the pharma companies may not have full exposure to what’s out there – in many cases they may not know that the ideal solution is already available waiting to be adopted, so it’s critical for technology developers to increase awareness of what’s available. On top of that, pharma’s conservative nature plays a role. The classic approach has led to lifecycle strategies where pharma progresses to initial approval and launch with an inferior delivery system, only considering advanced systems later, rather than implementing superior solutions, such as dual-chamber systems, initially.
If we’re going to see wide-scale adoption of dual-chamber technologies, we’re going to need to develop and demonstrate supply chain readiness – for pharma to proceed in the required investments to implement these systems, they will want to know that there is a direct and believable path with the device ready to go and the supply chain established and secure.
Q What role has Credence assumed in enabling dual-chamber solutions to address these unmet needs?
A At our core, Credence MedSystems is an innovator and supplier of problem-solving injectable drug delivery systems. So, our first role is in developing and providing a great dual-chamber platform that addresses the needs of our pharma customers and end-users.
But a delivery system without a means of turning it into a combination product is only half of a solution. Therefore, Credence is taking the lead in enabling development of the dual-chamber fill-finish supply chain readiness by engaging with leading equipment manufacturers and CDMOs (Figure 4). That’s a key part of my role at Credence – I collaborate and partner with CDMOs and equipment manufacturers to make sure that dual-chamber capacity is being built up.

Figure 4: Credence is an enabling link for implementation of dual-chamber fill-finish.
These kinds of partnership are critical for bringing pharma on board. By establishing partnerships and building up supply chain readiness, we’re reducing the risk for pharma companies, which is one of the key barriers stalling pharma from implementing dual-chamber systems more broadly – it’s a classic chicken and egg scenario.
Exactly to this point, we are so pleased to announce a collaboration with SMC Pharma Services for implementation of dual-chamber fill-finish readiness. The first focus is on implementing the capability for clinical supply, followed by commercial implementation. We will be ready to share more information soon. By taking on some of the initial investment and demonstrating the path to dual-chamber filling, we are making it that much easier for pharma to understand the business case. This approach also enables us to engage with multiple pharma companies, establishing what I call a syndicate relationship, where several partners come together for mutual benefit.
Credence’s technology is focused on the concept of “Innovation Without Change”. This means providing new solutions using existing infrastructure and components. Innovation Without Change is about enabling pharma to use their preferred off-the-shelf primary containers, which Credence then turns into dual-chamber systems. It is also about enabling these delivery systems to be filled by a network of CDMOs to unlock the potential in the market.
It’s going to take successes from the early adopters to fully shift the dial. Once those products start gaining traction in the market, I think we’ll see pharma’s comfort expand. Then, when that awareness starts to build and other pharma see that dual chamber is here, there’ll be a big shift towards dual-chamber solutions.
Q Finally, in summary, what would you say is the key takeaway message you’d like to send to pharma regarding adopting dual-chamber technology?
A First and foremost, Credence’s dual-chamber systems are elegant and flexible solutions that simplify user steps, offer end-of-dose user cues, enhance dosing accuracy and provide safety features. Second, because the Dual Chamber employs very simple user steps, it promotes adherence and enables at-home administration of complex drugs and encourage patients to keep up with their treatment. This is not only better for patients but better for pharma, as it provides a key differentiator in a crowded market.
Furthermore, it’s not just one product but rather a foundation for a series of presentations to support various use cases and lifecycle strategies. Credence offers a flexible range of adjacent technology solutions, including the manual injector and autoinjector. Looking to the future, we have the scope to expand this offering into additional technologies, such as connectivity. This means that we can meet a wide range of patient needs and tailor our technology to address the challenges of the specific application.
The last major takeaway is our collaboration with SMC that I touched on previously. This partnership is a great step in enabling pharma to implement dual-chamber solutions. We welcome pharma partners to collaborate with us in a joint partnership with SMC. It’s a proud and exciting moment for us. All we have talked about is consistent with Credence’s ongoing efforts to solve challenges facing injectable drug delivery, on behalf of our pharma customers and the patients and end users we all endeavour to help.
To learn more about the Credence Dual Chamber system, visit: www.credencemed.com/dual-chamber.
Read the recent press release announcing the partnership between Credence and SMC Ltd, here.

