To Issue 177
Citation: Balson M F M, “Delivering Complexity: Device Considerations for Two-Component Injectable Formulations”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 32–37.
María FM Balson weighs up strengths and limitations when developing devices for lyophilised injectables, exploring their growing demand and the impacts of formulation, device type and design on the feasibility for bringing a device to market to deliver these essential therapies.
Since the 1980s, when modern-day prefilled syringes (PFSs) and intravenous (IV) bags became prevalent, injectable drug delivery has steadily moved towards ready-to-use formats and integrated devices – as evidenced by the widespread adoption of self-injection devices such as autoinjectors and pen injectors.
Human factors considerations, now recognised as integral to safe and effective use of such drug-device combination products, have driven a clear trend towards simpler, more automated solutions with fewer use steps. This shift has enabled at-home care for more therapies than ever before – a key development given the growing strain on healthcare systems.
Nevertheless, the delivery of certain drugs, such as lyophilised injectables, often remains burdensome and dependent on administration by specially trained professionals. As injectable therapies evolve and become more complex, unique challenges and opportunities have emerged.
TWO-COMPONENT INJECTABLES ON THE RISE
Let’s define two-component formulations as those consisting of two parts that, for stability or other reasons, must be kept separate throughout the product’s shelf-life, and are delivered together at the point of administration. The two constituent parts may be a solid drug and a liquid solvent or diluent (e.g. sterile water for injection) that must be mixed thoroughly before use. Alternatively, both constituents may be liquid, in which case they may either require mixing prior to delivery or be delivered sequentially (Figure 1).
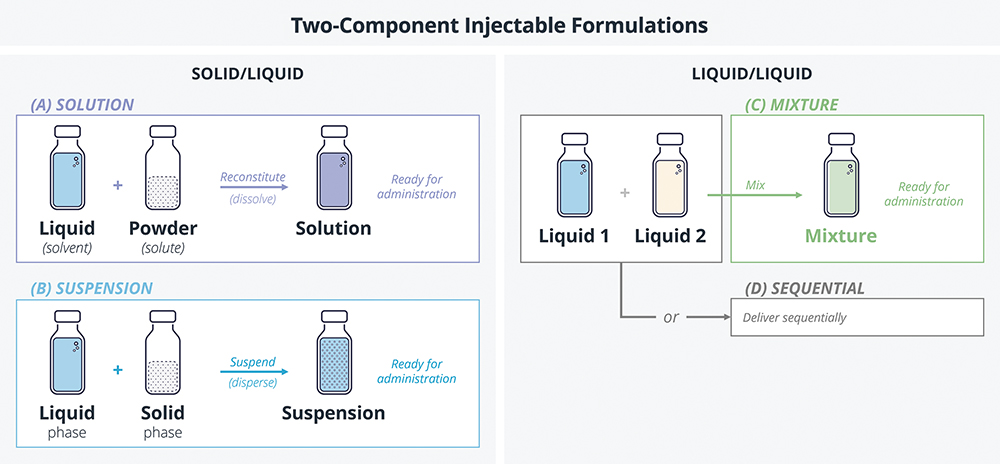
Figure 1: A simplified model of two-component injectables, classified according to the state of matter of constituent parts.
The Archetype: Solid/Liquid Reconstitution
Reconstitution is the process of adding a liquid solvent to a solid medication to dissolve it and form a solution. This may be required, at point of use, when a drug is unstable in liquid form and must therefore be stored dry. In such cases, the formulation is often filled as a liquid and then lyophilised (freeze dried) in situ. Alternatively, it may be manufactured and handled as a powder.
Freeze drying is an effective way to increase formulation stability. For small molecules, it can eliminate the need for cold-chain storage. For biologics (especially those that are large, complex or prone to aggregation) it can be a necessity in order to achieve an acceptable shelf-life.
“LYOPHILISED FORMULATIONS NOW REPRESENT OVER 30% OF ALL FDA-APPROVED PARENTERAL MEDICATIONS – AND DEMAND FOR LYOPHILISED PARENTERAL PRODUCTS IS INCREASING.”
Lyophilised formulations now represent over 30% of all US FDA-approved parenteral medications1 – and demand for lyophilised parenteral products is increasing, as evidenced by past drug approvals (~35 such drugs were approved by the FDA each year over the past decade, compared to ~12 per year in the decade prior2). Considering lyophilised parenterals approved in 2023, oncology and infectious disease indications represented the largest share, together accounting for ~75% of total approvals.2
As lyophilisation is on the rise, so too are devices to simplify reconstitution. A wide range of solutions are available beyond the well-established vial-and-syringe method – from primary container adaptors to dual-chamber systems.
Solid/Liquid Suspensions
Suspensions are a dosage form in which insoluble solid particles are mixed into a liquid medium. They enable delivery of insoluble drugs and can be used to formulate long-acting injections. Suspensions may be supplied as separate wet and dry components (in which case the liquid phase is added to the solid phase and mixed prior to administration) or in a single primary container that is shaken to resuspend.
While solutions can readily be reconstituted with gentle swirling, suspensions usually need a greater energy input to achieve even mixing – the required amount varies greatly depending on the chemical and physical properties of the formulation. In some cases, vigorous shaking is insufficient and benchtop equipment, such as a vortex mixer, must be used.
Given sufficient energy input, the particles will be uniformly dispersed within the liquid, however the resulting mixture will be heterogenous and unstable; it will eventually settle. Therefore, suspensions must be thoroughly mixed immediately before use. Inconsistent dispersion can lead to inaccurate dosing or needle clogging – persistent challenges for device integration.
Injectable suspensions are becoming more prevalent, particularly for severe chronic conditions such as schizophrenia and HIV,3,4 where extended-release formulations are of particular value and which are often reliant on a suspension format to produce a long-acting depot. When formulated as separate wet and dry components, these products largely rely on vial-and-syringe or vial-adaptor workflows, with the occasional exception, such as Eligard’s (leuprolide acetate, Tolmar) reciprocating syringes, or the Abilify Maintena (aripiprazole, Otsuka) dual-chamber syringe.3,4
Liquid/Liquid Mixtures
Injection of two-liquid mixtures is rarer but not unheard of. Two liquids may be mixed and delivered together out of:
- Necessity: When a formulation consisting of two fluid phases is unstable in mixed form, but must be mixed prior to injection in order to achieve the intended therapeutic effect (e.g. API and polymer solutions that mix to form a long-acting depot).
- Convenience: If two liquid formulations are frequently administered together, such as in combination vaccines, pharma companies may choose a dual-chamber presentation over developing a coformulation, such as with Vivaxim (typhoid and hepatitis A, Sanofi).6 In this case, mixing isn’t necessary but rather a side effect of leveraging mature dual-chamber systems (which mix the two liquids prior to administration) rather than betting on more niche sequential delivery technology.
Sequential Delivery of Two Liquids
Sequential delivery of two different liquids through a single needle or injection port has been proposed for combination therapies, as well as for IV drug administration through a vascular access device (with the drug preceded, or followed, by a catheter flush).7
While there are several delivery technologies in development that might enable these use cases, only one combination product in this category is on the market at the time of writing, according to data from PharmaCircle. The DuoDote emergency-use autoinjector (Meridian Medical Technologies (now part of Kindeva), based on a custom primary container, sequentially injects atropine and pralidoxime chloride. It is approved for treatment of nerve agent or insecticide poisoning.
“CHOOSING THE RIGHT DEVICE FOR A TWO-COMPONENT INJECTABLE IS OFTEN AN EXERCISE IN TRADE-OFFS, HIGHLY DEPENDENT ON THE PROPERTIES OF THE FORMULATION ITSELF, INDICATIONS FOR USE AND THE STAGE OF DEVELOPMENT.”
CHOOSING THE RIGHT DEVICE
Choosing the right device for a two-component injectable is often an exercise in trade-offs, highly dependent on the properties of the formulation itself, indications for use and the stage of development. Hereafter, this article will assume that a two-component injectable consists of separate wet and dry constituents that are reconstituted prior to injection, unless otherwise stated. This section will briefly cover the range of available technologies, and factors to consider when it comes to device selection.
Vial and Syringe: Trusty but Burdensome
Two-component injectables are often supplied in vials, with off-the-shelf (OTS) needles and syringes used for fluid transfer and subsequent injection (Figure 2). By leveraging mature primary containers and fill-finish technologies, this approach benefits from low unit cost and a robust supply chain. It is also extremely versatile, with fewer restrictions on formulation volume and viscosity compared with alternatives, the ability to accommodate different doses in a single stock keeping unit, and no need for device-specific training.
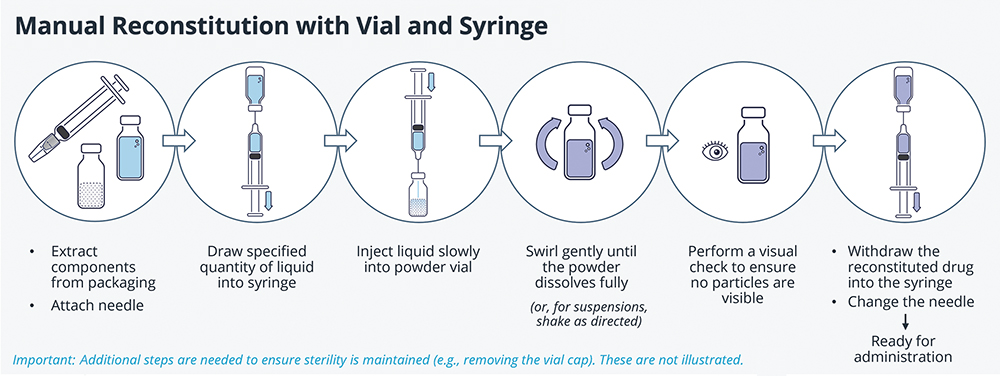
Figure 2: A summary of steps required for manual reconstitution using vials and syringes.
On the other hand, the process is onerous and a high degree of technical expertise is required to perform all steps correctly. Dose accuracy is highly dependent on the user, and there is a greater risk of contamination and sharps injury compared with other methods, meaning that this type of system is typically limited to trained staff in clinical settings. Moreover, some drug wastage is inevitable, with vials often overfilled by 10–20% to ensure that a full dose can always be drawn.
Devices to Simplify the Reconstitution Process
Given the growing prevalence of two-component injectables and the limitations of the established vial-and-syringe method, it is no surprise that a wide range of specialist devices have been developed to aid reconstitution. Figure 3 illustrates some of the solutions available.
- Primary Container Adaptors: Co-packaged with standard prefilled primary containers, these allow for drug components to be accurately pre-dosed during manufacturing, while maintaining low device and fill-finish costs.
- Integrated Manual and Automated Systems: Some of these leverage standard OTS containers, while others are designed around bespoke primary containers (e.g. dual-chamber cartridges).
– Integration of device components reduces the number (and sometimes complexity) of use steps, reducing the burden of use and the likelihood of errors.
– Automated devices take this further by incorporating mechanisms in the design (such as springs or electronics) to enable reconstitution and/or delivery with minimal user input.
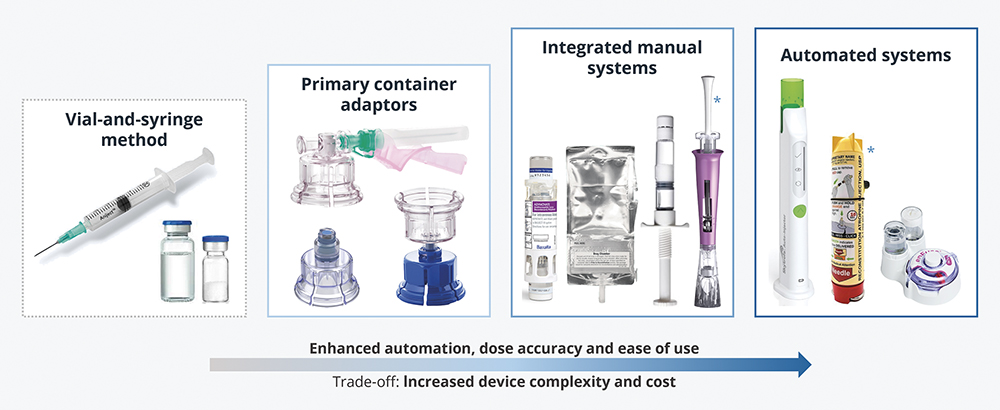
Figure 3: Examples of reconstitution devices for intravenous, intramuscular and subcutaneous administration. Devices marked with an asterisk are in development at the time of writing; the others are on the market. Note that prefilled dual-chamber systems can fall within the “integrated manual” or the “automated” categories, depending on device function.
Horses for Courses: Different Drugs Have Different Needs
When choosing a device, key trade-offs include cost, time to market, dose accuracy and ease of use. Consider:
- Properties of the Formulation: All reconstitution devices have their strengths and limitations; the choice of device must be compatible with the needs of the formulation. For example, dual-chamber PFSs are limited to products with relatively low volumes that reconstitute readily.
- Use Case and Dose Accuracy: The choice should be made with the final user in mind; integrated and automated systems greatly simplify usage, making accurate reconstitution accessible to users with less technical expertise (e.g. patients in the home setting).
- Supply Chain Implications: The choice of primary container is the single most important factor influencing development timeline and manufacturing cost of the device. Dual-chamber fill-finish is highly complex; expertise is rare and CMO capacity limited.
- Stage of Drug Development: Priorities differ depending on the stage of development. For example, a novel drug in clinical trials may benefit from the use of vials, since they offer flexible dosing and use only OTS components, whereas more integrated systems may be introduced post-launch to encourage wider adoption.
DUAL-CHAMBER DELIVERY SYSTEMS
Prefilled dual-chamber systems (DCSs) are “all-in-one” devices built around bespoke primary containers, designed to simplify the reconstitution and delivery of two-component injectables. This final section delves deeper into this device category –strengths, limitations and key design considerations.
Anatomy of a Dual-Chamber System
In a DCS, the primary container consists of a barrel (typically made of glass) divided into two chambers by a central stopper. This barrier keeps the drug components separate from each other throughout storage. Once the DCS is activated, a bypass mechanism allows fluid to flow from the back (wet) chamber into the front (typically dry) chamber (Figure 4).
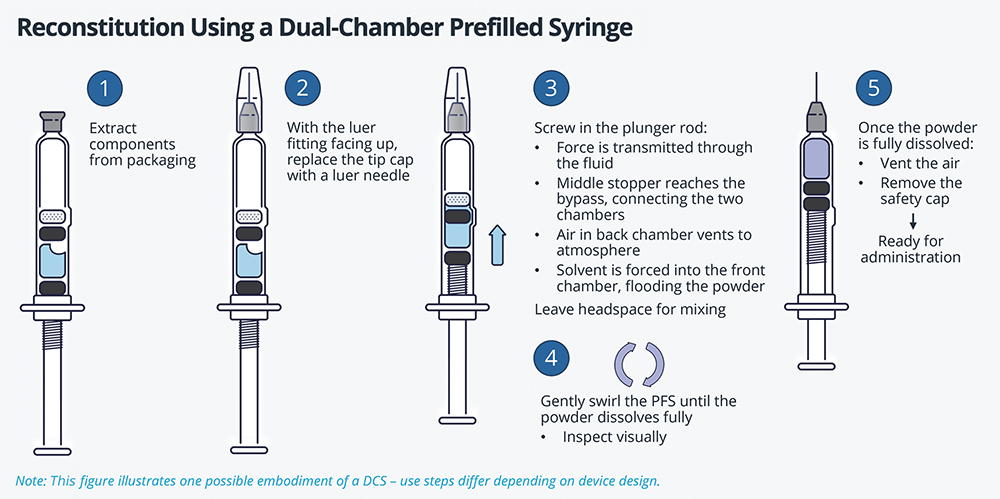
Figure 4: Use steps and function of a typical DCS embodiment. Note that the linear application of force causes the bypass mechanism to activate, opening a fluid path that connects the two chambers.
DCSs vary in type of closure and bypass:
- The closure can be PFS-style or cartridge-style (Figure 5).
- The bypass is usually external (a blister bypass), but can also be internal (such as the multi-groove design of the Genotropin MiniQuick (somatropin, Pfizer) – Figure 5, Device 5). Note that an internal bypass allows the use of standard syringe or cartridge tubs, which is advantageous for manufacturing. Emerging designs, such as Credence MedSystems’ fenestrated needle bypass, also have the additional benefit of being compatible with OTS syringes.
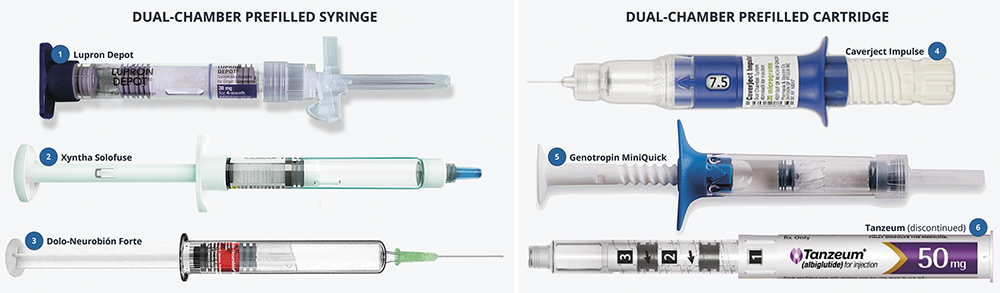
Figure 5: Approved DCS products (all marketed, bar Tanzeum (albiglutide, GSK), which has been discontinued). Left: dual-chamber prefilled syringes. Right: integrated injection devices built around dual-chamber cartridges. Device 1 contains a lyophilised suspension; Devices 2, 4, 5 and 6 contain lyophilised solutions; and Device 3 contains two liquids for co-administration.
Bespoke Primary Containers: A Double-Edged Sword
Like other specialist reconstitution devices, DCSs make administration of two-component injectables accessible to a wider range of users and care settings. They require less technical expertise to use accurately and consistently, with fewer and simpler handling steps, pre-measured drug components and reduced sharps exposure.
“THANKS TO THIS DESIGN, DCSS CAN READILY BE INTEGRATED INTO DEVICES WITH ENHANCED USABILITY AND/OR ADVANCED FEATURES.”
However, their unique strength lies in their form factor – the single barrel with a bypass that can be activated with a co-linear application of force (so both mixing and delivery are done by pushing on the rear plunger in a straight motion). Thanks to this design, DCSs can readily be integrated into devices with enhanced usability and/or advanced features. For example:
- Xyntha Solofuse (antihemophilic factor, Pfizer), an easy-to-use device with a simple finger flange (Figure 5 Device 2).
- Caverject Impulse (alprostadil, Pfizer), an integrated manual system with dose selection capability (Figure 5 Device 4).
- The reusable Skytrofa autoinjector (lonapegsomatropin-tcgd, Ascendis), pictured in Figure 3 with the green needle guard.
The flip side of the form-factor coin is that complexity is pushed into the manufacturing and filling process. Fill-finish for these devices requires specialist equipment and know-how (as noted above, expertise is rare and capacity is limited) and lyophilisation is inherently less efficient in the dual-chamber geometry compared with vials (smaller batches, poorer energy transfer, longer cycle times6). It all adds up to greater up-front investment and time-to-market, higher unit cost and a restricted supply chain.
For this reason, DCSs have so far been limited to premium value products, such as those used to treat rare diseases (e.g. haemophilia, growth hormone deficiency) or those that solve complex or critical clinical challenges (e.g. unmet needs, home care).6
Design Considerations
Current marketed DCSs have inherent technical limitations that impact formulation compatibility and device design. For example:
- Capacity is Limited to ~4 mL Total Reconstituted Volume: Headspace in the front chamber must be sufficient to accommodate the initial plunger stroke required to open the bypass, both drug components and additional room for swirling and mixing. Therefore, there is a limit to how much can be delivered with these devices before they become too large to be practical.
- Venting and Orientation are Important: There usually needs to be a path to atmosphere during mixing to avoid pressure build-up in the front chamber (if there is a large amount of headspace in the powder chamber, this may not be required). In all cases, excess air must be vented prior to injection, which can be challenging and requires careful handling, as the device must be kept upright whenever there is a path to atmosphere to avoid drug spilling through the needle.
- Plunger Motion Must be Well-Controlled: When the bypass opens, the pressure in the system drops sharply. Unless the plunger’s forward motion is well-controlled, there is a risk of prematurely locking out the fluid path, which would prevent the liquid in the back chamber from being fully incorporated into the mixture. To prevent this, many devices incorporate a screw mechanism that enforces a slower twist-to-mix action.
- They Are Best Suited to Lyophilised Formulations That Are Readily Reconstituted with Gentle Swirling: Suspensions can only be delivered if the energy required to suspend is low. In addition, sequential delivery is not possible without specialised valve design (some mixing will always take place with the currently marketed DCSs). Finally, very particular considerations apply to the delivery of liquid/liquid mixtures – space is at an even greater premium, venting becomes critical and mixing performance varies widely depending on the specific device and formulation.
LOOKING AHEAD
Meeting the next generation of injectable delivery challenges will demand the best of device innovation, alongside advances in formulation and process development. As therapies grow more complex, the need for close cross-functional collaboration becomes increasingly critical.
Developers of combination products will continue to face trade-offs between usability, flexibility, cost and manufacturability. To navigate these successfully, device and formulation experts must work hand-in-hand with clinical, regulatory, commercial and access stakeholders. Working together, medicines can be delivered that are fit for purpose today and ready to meet the needs of tomorrow.
REFERENCES
- Kumar S et al, “Application of lyophilization in pharmaceutical injectable formulations: An industry and regulatory perspective”. J Drug Deliv Sci. Technol, 2024, Vol 100, art 106089.
- Gray J, “LyoHUB 2024 Annual Report”. PharmaHub, 2024.
- “Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations”. Web Page, US FDA, accessed Jul 2025.
- “Purple Book: Database of Licensed Biological Products”. Web Page, US FDA, accessed Jul 2025.
- “DailyMed: Prescription drug labeling and information.” US National Library of Medicine, accessed Jul 2025.
- Werk T et al, “Technology, Applications, and Process Challenges of Dual Chamber Systems”. J Pharm Sci, 2016, Vol 105, pp 4–9.
- Sousa et al, “Brief Report on Double-Chamber Syringes Patents and Implications for Infusion Therapy Safety and Efficiency”. Int J Environ Res. Public Health, 2020, Vol 17(21), art 8209.
Previous article
DIVERGENT DESIGN PHILOSOPHIES IN THE WEARABLE INJECTOR SEGMENTNext article
Early Insight: DuoVIAL® – PROTECT, MIX AND DELIVER
