To Issue 177
Citation: De Clercq K, Vasiev A, “Powder to the People: Accelerating Access to Lyophilised Therapies”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 38–43.
Kristien De Clercq and Dr Alex Vasiev discuss the challenges and recent advances in drying and reconstitution technologies, as well as how, while dual-chamber primary packaging offers a potential solution for simplifying reconstitution, it remains underused due to usability concerns and a prevailing industry preference for vial-based lyophilisation over alternative drying methods such as spray drying and powder filling.
LYOPHILISATION IN DRUG DEVELOPMENT
Lyophilisation, or freeze drying, is a widely used formulation strategy in drug development, particularly for biologics such as monoclonal antibodies, peptides and nucleic acids. These molecules often exhibit limited stability in liquid form, making lyophilisation an attractive solution. By removing water, the primary mediator of many degradation pathways, this process can dramatically extend shelf life, reduce storage constraints and simplify transport across diverse climates by minimising or eliminating cold-chain requirements.
In this low-temperature dehydration process, the product is first frozen and then exposed to reduced pressure, causing frozen water to sublimate directly from solid to vapour. If well controlled, this gentle method preserves the structural integrity and biological activity of sensitive compounds. Lyophilisation can be implemented at virtually any scale, allowing seamless progression from early laboratory experiments to clinical development batches and, ultimately, large-scale commercial production.
Liquid Versus Lyophilised Formulations
The decision to transition from a lyophilised product to a stable liquid formulation is commercially driven. Many companies launch with a lyophilised version to accelerate regulatory approval and establish a revenue stream. Later, if justified by market demand, they may invest in ready-to-use liquid formats or prefilled syringes as part of a lifecycle management strategy.
Liquid formulations simplify use by eliminating the reconstitution step, which is particularly valuable for home-use scenarios where mixing can be perceived as cumbersome or prone to error. However, developing a stable liquid formulation carries significant technical and regulatory risks. It requires extensive formulation research, stability (“stress”) testing, manufacturing process validation and sometimes additional clinical trials. For certain molecules, inherent instability in liquid form makes this route technically or commercially unfeasible.
“WHEN REFORMULATION IS IMPRACTICAL, A LYOPHILISED DRUG CAN BE PAIRED WITH A RECONSTITUTION DEVICE. THESE DEVICES STREAMLINE PREPARATION, REDUCE TRAINING REQUIREMENTS, IMPROVE PATIENT CONFIDENCE AND MINIMISE THE ERRORS ASSOCIATED WITH MANUAL MIXING – DELIVERING MANY USABILITY BENEFITS WITHOUT ALTERING THE DRUG ITSELF.”
When reformulation is impractical, a lyophilised drug can be paired with a reconstitution device. These devices streamline preparation, reduce training requirements, improve patient confidence and minimise the errors associated with manual mixing – delivering many usability benefits without altering the drug itself.
Challenges of Manual Reconstitution
For self-administered therapies, manual reconstitution can be difficult or frustrating for patients and caregivers. Typical steps include cleaning components to ensure sterility, connecting and disconnecting fluid paths, swirling or rolling to mix and judging whether or not the final dose is fully reconstituted. These long, complicated processes create many opportunities for use error, which can lead to incorrect dilution or incomplete reconstitution, directly affecting treatment efficacy.
Faced with these challenges, users may turn to shortcuts such as skipping steps that they don’t think are important or using the injection needle to access the vial, exposing them to further potential harms. All of these challenges are further multiplied if the patient population suffers from manual dexterity issues or other comorbidities that may affect their ability to complete the reconstitution process.
DUAL-CHAMBER DEVICES
Dual-chamber devices (DCDs) offer a significant advantage by performing aseptic fluid transfer and reconstitution inside the primary packaging (syringe or cartridge), thereby eliminating the risks of leakage and contamination during preparation. Several regulator-approved devices already incorporate this technology (Table 1).
| Drug | Company | Route | Primary Pack | Device | Year of Approval |
| Humatrope (somatropin) | Eli Lilly | SC | Cartridge | HumatroPen (Ypsomed) | 1987 |
| Genotropin (somatropin) | Pfizer | SC | Cartridge/Syringe | Genotonorm or Miniquick (Ypsomed and Pfizer, respectively) | 1995 |
| Cardizem (diltiazem) | Bioavial Pharmaceuticals | IV | Syringe | Lyo-Ject (Vetter) | 1996 |
| Saizen (somatropin) | Merck Serono | SC/IM | Cartridge | One-click pen (Haselmeier) | 1996 |
| Edex (alprostadil) | Schwarz Pharma | IC | Cartridge | Edex Injection Device (Schwarz, now UCB) | 1997 |
| NeoRecormon (epoetin beta) |
Roche | SC | Cartridge | Reco-Pen (Ypsomed) | 1997 |
| Caverject (alprostadil) | Pfizer | IC | Cartridge | Caverject Impulse (Pfizer) | 2002 |
| Xyntha Solofuse (antihaemophilic factor) | Pfizer | IV | Syringe | Lyo-Ject (Vetter) | 2014 |
| Tanzeum (albiglutide) | GSK | SC | Cartridge | LyoTwist® (Ypsomed) | 2014 |
| Abilify Maintena (aripiprazole LAI) | Otsuka Pharmaceuticals | IM | Syringe | Dual-Chamber Syringe (Arte/Otsuka) | 2014 |
| Bydureon (exenatide extended release) | AstraZeneca | SC | Cartridge | LyoTwist® (Ypsomed) | 2014 |
| Skytrofa (lonapegsomatropin-tcgd) | Ascendis Pharma | SC | Cartridge | Skytrofa Autoinjector (Phillips Medisize) |
2021 |
Table 1: Non-exhaustive list of devices using dual-chamber primary packaging.1,2 (SC – subcutaneous, IV – intravenous, IM – intramuscular, IC – intracavernous)
Despite these benefits, DCDs present their own usability challenges. While these devices may reduce the number of use steps in the preparation process, they may still contain tasks that could be confusing for users, such as combinations of pushing and screwing actions where use errors can still occur.3 Further to this, the larger size of some dual-chamber syringes may be daunting for patients.
From a product-design perspective, ensuring long-term moisture protection for a lyophilised cake places high demands on the stopper-to-container interface – requirements that are intensified in cartridge-based systems with more sealing points. After activation, the lyophilised powder must dissolve completely within a practical timeframe and without undesirable effects, such as foaming, particle formation or incomplete mixing. This makes certain device platforms unsuitable for formulations with inherently slow dissolution rates.
“TRANSFERRING A VALIDATED VIAL-BASED LYOPHILISED FORMULATION TO A CARTRIDGE OR SYRINGE FORMAT INTRODUCES ADDITIONAL REGULATORY AND CMC HURDLES, REQUIRING NEW STABILITY STUDIES AND MANUFACTURING VALIDATION.”
Transferring a validated vial-based lyophilised formulation to a cartridge or syringe format introduces additional regulatory and chemistry, manufacturing and control (CMC) hurdles, requiring new stability studies and manufacturing validation. Moreover, manufacturing DCDs adds complexity at the fill-finish stage – dedicated processing lines and specialised transport fixtures are needed, and freeze-drying performance in these formats has historically been limited by inconsistent heat transfer due to product residing too far from the heat-transfer surface and unfavourable surface-area-to-volume ratios.
These factors can prolong drying cycles and make it harder to achieve uniform cake quality.1 Specialised fixtures are necessary to support syringes during freeze drying, and cycle optimisation is essential to prevent cake collapse or loss of structure. Although technological advances have improved freeze drying in cartridges and syringes, industry experience and data remain more extensive for vial formats.
ALTERNATIVE TECHNOLOGIES
There are, however, a number of promising device and process technologies that, combined or in isolation, have the potential to reduce user burden when reconstituting drugs and usher in a new generation of combination products. Their successful adoption will require a multidisciplinary approach, as novel combination product development relies on a fundamental understanding of formulation, biopharmaceutical processing, reconstitution physics and fill-finish and device expertise.
Spray Drying
Another, though less widely used, approach to drying therapeutics is spray drying, which offers exceptional control over the dissolution properties of dry formulations by engineering particles through the kinetics of the drying process.4 Unlike freeze drying, spray-dried particles can be solidified in an amorphous state, improving the stability of fragile molecules and accelerating dissolution.
The particle structure can also be influenced by the balance between solvent evaporation and solute diffusion, described by the Péclet number:
- Low Péclet Number: Solutes remain evenly distributed, producing solid particles
- High Péclet Number: Solvent evaporation outpaces solute diffusion, resulting in surface enrichment and the formation of a shell.
Proteins and polymers tend towards the high Péclet number scenario, but this can be adjusted using other process parameters (Figure 1). By controlling solvent-drying kinetics, selecting appropriate excipients and adjusting spray-drying parameters, manufacturers can fine-tune several key characteristics:
- Particle surface area
- Stabilisation of an amorphous state (useful for both stability and dissolution)
- Particle density (e.g. solid, foamed or hollow)
- Barrier- or surface-modifying coatings
- Powder dispersibility and flowability
- Controlled drug-release profiles.
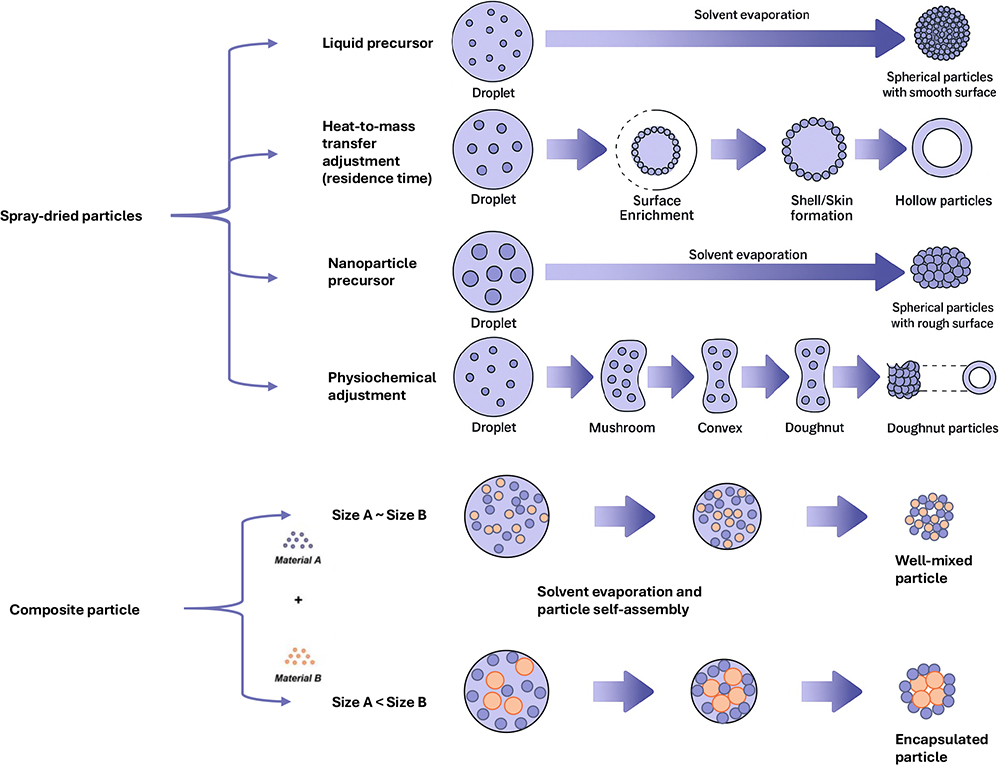
Figure 1: Illustration of the level of control possible over particle morphology using spray-drying process design.4
When optimised, spray drying can reduce reconstitution times from several minutes to mere seconds.5 This has the potential to significantly improve usability and adherence, even with existing device technologies, by removing some of the difficulties users face.
Because it is perceived as a harsher process than freeze drying, spray drying has traditionally been used for small-molecule drugs. However, it has been shown to be suitable for biologics, including complex biomolecules, such as immunoglobins and viral capsid proteins, due to evaporative cooling and the use of protective excipients, such as trehalose.
Aside from unfamiliarity, adoption for injectable formulations has been limited by practical challenges, including the availability and maturity of aseptic spray drying and powder handling, high cost of physical losses at small scale (up to 50%), and the limited number of CDMOs available with experience in this area.
Today, some of these barriers, such as aseptic handling and powder filling, are gradually diminishing, increasing the accessibility of this technology. However, as with lyophilisation, spray drying requires the development of an appropriate formulation – a process that can be time-consuming and demands specialised expertise.
“SEVERAL NOVEL DRUG-DEVICE COMBINATION PLATFORMS AIM TO ACHIEVE THIS BY AUTOMATING SOME OR ALL PREPARATION STEPS. THESE SYSTEMS REDUCE USER BURDEN, LOWER THE RISK OF USE ERRORS AND IMPROVE OVERALL USABILITY COMPARED WITH MORE MANUAL METHODS.”
Alternative Reconstitution Device Technologies
A different approach to simplifying and standardising the process of reconstitution is to design around the issues. Several novel drug-device combination platforms aim to achieve this by automating some or all preparation steps. These systems reduce user burden, lower the risk of use errors and improve overall usability compared with more manual methods.
Windgap Medical’s Large Volume Dual Cartridge platform employs a side-by-side configuration of two standard single-chamber cartridges – one containing the diluent and the other the lyophilised drug product. Reconstitution occurs through cyclic fluid transfer between the cartridges, ensuring thorough mixing prior to administration. Two variants are offered – a touch-activated design and a compressed-gas-driven version, the latter capable of delivering large volumes and handling highly viscous formulations.
Eveon’s Intuity® Ject MX also uses cartridges as primary packaging and achieves reconstitution via cyclic fluid transfer between two containers. However, it replaces manual or gas-driven actuation with an electromechanical mixing mechanism. This automation standardises the mixing process and allows for connectivity features that enable data capture and adherence monitoring. Additionally, Eveon offered the Intuity® Mix, a piston-pump-based, non-portable, fully automated system intended for clinical or pharmacy use. (Note from ONdrugDelivery: As of April 2024, Eveon is in formal insolvency proceedings in France.)
Enable Injections’ EnFuse® system follows a different approach. While currently marketed versions are designed for liquid formulations, such as Empaveli® (pegcetacoplan, Apellis Pharmaceuticals, Waltham, MA, US), a variant exists that performs reconstitution immediately before pump filling. This method bypasses the challenges associated with in situ lyophilisation and container compatibility by connecting directly to a standard lyophilised powder vial. EnFuse uses an internal mechanical pumping system for fluid transfer and mixing, enabling it to handle a wide range of formulation viscosities.
Some systems address specific aspects of vial usability by automating diluent transfer but without automating mixing, transfer or delivery. Their vial-based format is generally bulkier than cartridge-based platforms and offers fewer opportunities for delivery device integration. However, it provides flexibility in dosing and route of administration, making these systems well suited to formulations with straightforward dissolution profiles or varied dosing regimens, while being less appropriate for tackling challenges such as high viscosity.
Baxter’s BaxJect III®, marketed for use with ADYNOVATE® (PEGylated antihaemophilic factor, Takeda Pharmaceuticals), uses two standard vials: one for diluent and one for lyophilised powder, connected via a sterile transfer device. Reconstitution is driven by a pressure differential between the vials, without an active mixing mechanism. The user must manually withdraw the prepared solution into a syringe through a side port.
DuoJect’s (Bromont, Canada) INTERVIAL™ family of devices, including PENPREP EVO™, approved for use with SAIZEN® (somatropin, Merck), combines the diluent and lyophilised powder within a single device. Mixing is manual, and the user then draws up the dose after reconstitution is complete.
Pfizer’s Act-O-Vial® offers a simple mechanical method to simplify reconstitution. It integrates the diluent and lyophilised drug in a single vial assembly separated by a rubber stopper. Pressing the stopper releases the diluent into the powder chamber, allowing mixing before withdrawal for injection.
Innovation in this space benefits most from partnerships that bridge formulation science, human factors expertise and engineering design into a single integrated development. By combining deep understanding of the drug product with insight into user needs and awareness of enabling technologies, it is possible to streamline reconstitution, reduce complexity and unlock the next generation of combination products. With unmet market need still evident, this remains a fertile area for innovation and a compelling opportunity for growth.
CONCLUSION
Lyophilisation remains the preferred solution for stabilising sensitive biologics, offering substantial benefits in shelf-life extension, storage flexibility and global distribution. However, the shift from traditional vial formats to integrated, patient-friendly delivery systems is an area rich with innovation. Dual-chamber primary packaging and in-device reconstitution platforms promise simpler preparation and fewer use errors, yet they bring their own user and technical challenges, such as larger size, potentially confusing use steps, freeze drying within constrained geometries, ensuring rapid and complete dissolution and maintaining long-term stability.
Emerging alternatives, such as spray drying, present new opportunities to tailor powder characteristics and enhance reconstitution efficiency. Still, historical barriers, including the complexity of aseptic powder filling and high material losses at small scale, have limited broader adoption. Advances in device technology are also driving progress, with platforms that aim to automate some or all steps of the reconstitution and delivery process.
Ongoing innovation in both drying and delivery technologies is fuelled by the need to improve patient convenience, adherence and therapeutic outcomes. The widespread adoption of on-device reconstitution will depend on carefully balancing formulation stability, manufacturing feasibility and human factors. The potential reward is significant – expanding access to complex biologics in formats that are both highly effective and intuitive to use across diverse markets and therapeutic areas. Achieving this will require multidisciplinary collaboration, less-siloed development approaches and strategic partnerships that unite expertise in formulation, engineering, and user-centric design.
REFERENCES
- Werk T et al, “Technology, Applications, and Process Challenges of Dual Chamber Systems”. J Pharm Sci, 2016, Vol 105(1), pp 4–9.
- Ingle RG, Fang W-J, “Prefilled dual chamber devices (DCDs) – Promising high-quality and convenient drug delivery system”. Int J Pharm, 2021, Vol 597, art 120314.
- LaRue S, Malloy J, “Evaluation of the Dual-Chamber Pen Design for the Injection of Exenatide Once Weekly for the Treatment of Type 2 Diabetes”. J Diabetes Sci Technol, 2015, Vol 9(4), pp 815–821.
- Nandiyanto ABD, Okuyama K, “Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges”. Adv Powder Technol, Vol 22(1), pp 1–19.
- Tiene G, “Spray Drying Enhances Solubility and Bioavailability”. Pharma Manufacturing, Jan 2017.

