To Issue 177
Flippe M, Lelias S, “Reimagining Dual-Chamber Injection: De-Risking a Novel Valve Component for Use with Standard Syringe Systems”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 54–61.
Marc Flippe and Sophie Lelias introduce a novel valve component designed to enable dual-chamber functionality within standard syringe and autoinjector platforms. Developed through a comprehensive modelling and development framework, the BD dual-injection syringe system provides a scalable solution to dual-chamber injection that reduces the burden of change by preserving compatibility with established container formats and device architectures.
As the pharmaceutical industry continues to explore innovative drug formulation and delivery strategies, the co-formulation of drugs has emerged as a compelling approach, particularly for fixed-dose combinations of synergistic therapies targeting distinct mechanisms of action. While this strategy is well established for small molecules in areas such as infectious disease, diabetes and neurological diseases, its application to biologics is currently gaining momentum.1,2 The 2020 US FDA approval of the first co-formulated monoclonal antibodies marked a significant milestone, opening the door to broader application of co-formulated drugs across the global development pipeline.
Such co-formulations can offer multiple advantages, including enhanced therapeutic efficacy, reduced adverse effects, fewer required injections, cost efficiencies and intellectual property protection.1–3 For example, using a combination of antibodies could offer a more effective therapeutic intervention, as multiple epitopes can be addressed within a single drug product.3 However, these complex mixtures may also introduce significant development risks, including cross-reactivity, physical and chemical instability, safety concerns and complex analytical characterisation.1–3 This may be especially true for protein formulations with different pHs, excipient types and ionic strengths.1
An alternative to co-formulation may be dual injection, allowing for co-administration of two liquid drugs while maintaining separation until use. This approach may avoid the risks associated with the interaction of two drug formulations prior to use while preserving the benefits of combination therapies. Although several dual-injection solutions exist, many require specialised container formats, new materials and/or significant changes to device system architecture, limiting their compatibility with standard container and device platforms and increasing drug-device development complexity.
“THE BD DUAL-INJECTION VALVE FEATURES AN EXTERNAL SHAPE DESIGNED TO SUPPORT COMPATIBILITY WITH EXISTING SYRINGE SYSTEMS, AND INTEGRATES A NOVEL SLITTED VALVE ENGINEERED TO MAINTAIN SEPARATION OF TWO LIQUID DRUGS IN A STANDARD SYRINGE UNTIL USE.”
To address these challenges, BD has reimagined dual injection through the development of a novel valve component designed to provide the functionality of a dual-chamber system within existing prefillable syringe and autoinjector devices. Using an incremental innovation approach, this solution builds on known materials and device architectures to minimise risk for pharmaceutical developers. The BD dual-injection valve features an external shape designed to support compatibility with existing syringe systems, and integrates a novel slitted valve engineered to maintain separation of two liquid drugs in a standard syringe until use.
To de-risk the development and integration of this novel component into existing delivery platforms, BD employed a comprehensive modelling and simulation framework. This approach confirmed that dual-chamber functionality can be achieved with the BD dual-injection valve within standard prefillable syringe systems without requiring changes to the primary container, or major changes to the delivery device components or established processes, offering a streamlined and de-risked solution for pharmaceutical developers pursuing dual-injection delivery.
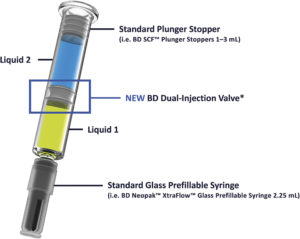
Figure 1: BD dual-injection syringe system*.
OVERVIEW OF THE BD DUAL-INJECTION SYRINGE SYSTEM
As a global leader in prefillable syringes and drug delivery systems, BD has drawn on its extensive expertise to develop the BD dual-injection syringe system (Figure 1). This system is designed to enable dual-injection delivery of two liquid drug formulations while maintaining compatibility within standard prefillable syringe and autoinjector systems.
At the core of this system is a novel valve component that maintains physical separation of the two liquids within a single syringe until use. Upon activation, Liquid 1 and Liquid 2 are administered, with Liquid 2 passing through the valve. This enables complete dual injection without the need for a bypass channel or specialised container geometry, meaning that it can be used in standard glass prefillable syringe, such as the BD Neopak™ Glass Prefillable Syringe platform.

Figure 2: Top view of the BD
dual-injection valve*.
The dual-injection valve component is designed with an external shape that eases syringe system integration and processing on existing pharmaceutical manufacturing lines. It is designed to provide dual-chamber functionality to a syringe system, isolating the two drugs prior to delivery, and incorporates a central slitted valve (Figure 2), engineered to open during injection, allowing for a controlled flow. Uniquely, the valve component is functionally symmetrical, simplifying orientation during automated handling. Furthermore, it uses well-characterised materials and validated packaging and sterilisation processes, thereby reducing the regulatory and operational risks typically associated with adopting a new component.
DE-RISKING DEVELOPMENT WITH MODELLING AND SIMULATIONS
A critical objective in the development of the BD dual-injection valve and syringe system was to ensure reliable dual-chamber functionality without requiring any significant changes to the primary container platform, standard autoinjector systems or existing fill-finish operations. To achieve this, BD applied a rigorous design approach grounded in advanced modelling, simulation and iterative analyses (Figure 3).
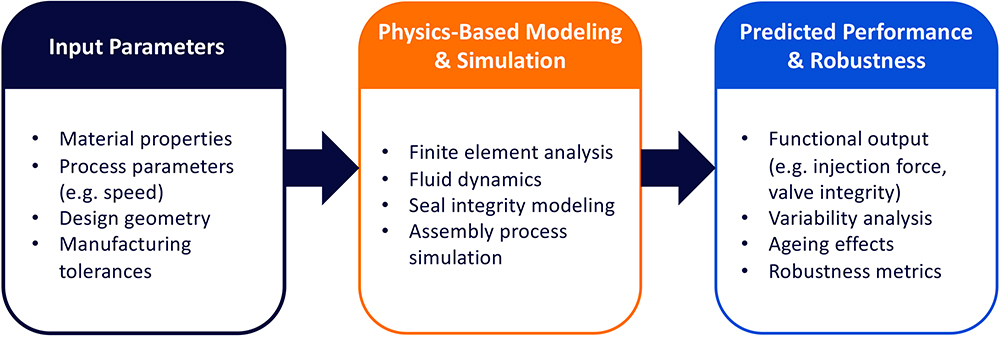
Figure 3: High-level design approach to de-risk BD dual-injection valve design.
The development framework began with the definition of key input parameters, including material properties, design geometry, customer-process parameters and manufacturing tolerances. These inputs were used to drive a suite of physics-based simulations, ranging from finite element analyses to fluid dynamics to seal integrity and assembly modelling. Together, these tools enabled BD to predict and optimise system design and behaviour under a wide range of real-world conditions.
By integrating these models into a unified framework, BD was able to evaluate key performance metrics, such as injection forces, valve actuation, fill volume accuracy and robustness, across various environmental and mechanical stressors. This predictive capability was essential for de-risking the design and ensuring that the BD dual-injection valve and syringe system could deliver reliable, reproducible performance while maintaining compatibility with existing manufacturing processes and autoinjector platforms.
Fill-Finish Modelling
To de-risk the fill-finish processability of the BD dual-injection valve and syringe system, BD implemented a comprehensive stack-up modelling approach that integrated both dimensional and functional design parameters. This methodology incorporated variability assessments to define maximum injectable dose and required fill volumes, including overfill margins, accurately by triangulating finite element modelling inputs.
The modelling also enabled precise prediction of the positions and dynamic movements of both the novel valve (Figure 4) and the plunger stopper throughout the anticipated drug-device combination product lifecycle. Furthermore, it accounted for environmental changes, including pressure and temperature variations, during fill-finish operations or during air shipment, ensuring robust system behaviour under real-world conditions.
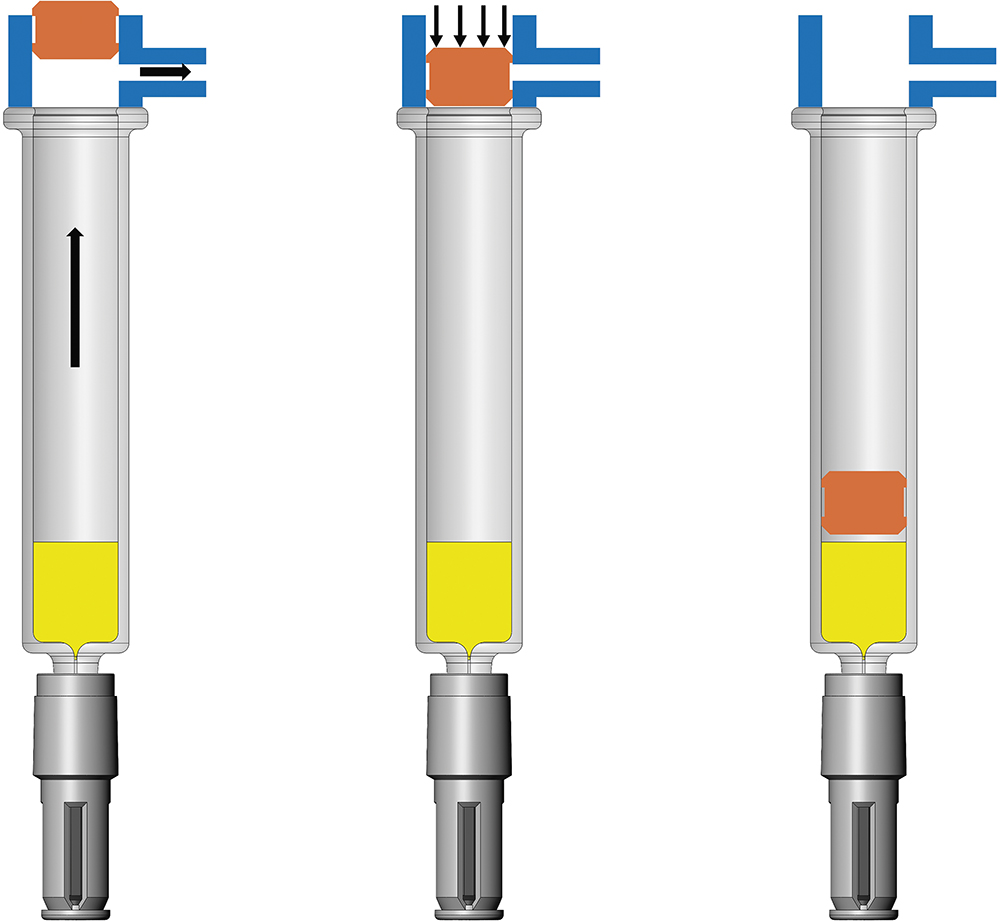
Figure 4: Schematic view of the dynamic movement during the stoppering step for the BD dual-injection valve component.
Additionally, the framework supported key integration calculations, such as the plunger stopper’s position before and after injection, reinforcing confidence in system performance from filling to transport and final injection.
FEA Simulations
Finite element analysis (FEA) simulations were employed to analyse and optimise the design within a defined space, ensuring that functional performance is maintained throughout the final drug-device combination product lifecycle. The accuracy of these simulations was verified by BD through targeted physical testing, providing a high level of confidence in the predictive outcomes.
This approach enabled detailed characterisation of component behaviour within the prefillable syringe system and its interaction with the final delivery device, such as a standard disposable autoinjector. These finite element simulations were particularly critical for sizing and evaluating the novel valve component, where certain behaviours may be difficult to observe or quantify through conventional testing alone.
Fluidic Modelling
Fluidic modelling was used to analyse flow dynamics and back-pressure behaviour throughout the valve featuring complex, pressure-driven geometry. This approach enabled detailed evaluation of the valve’s performance across the full range of product use conditions, such as during autoinjector-based injection. The modelling captured the entire sequence, from initial pressure build up on the valve surface, through valve opening to complete liquid transfer (Figure 5). This modelling proved to be essential for optimising the valve geometry and ensuring consistent performance under varying mechanical pressures.
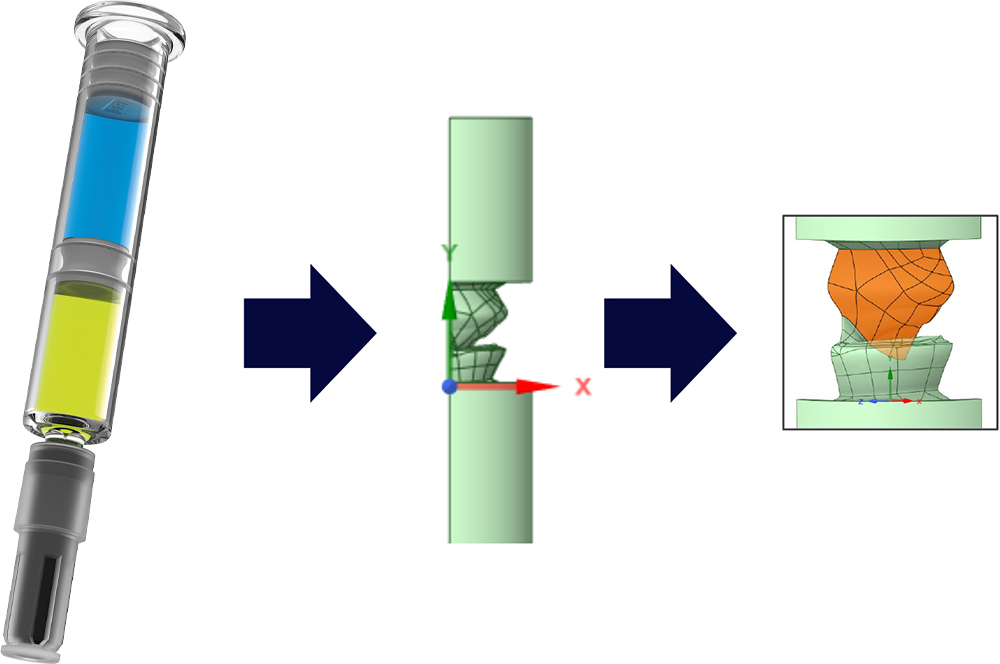
Figure 5: Example of fluidic simulation of the BD dual-injection valve and syringe system.
“BD IS ABLE TO DRAW ON DECADES OF EXPERTISE IN RUBBER COMPONENT DEVELOPMENT AND OPTIMISATION ACROSS ITS VAST PRODUCT PORTFOLIO, WHICH GIVES IT A ROBUST UNDERSTANDING OF THE PHYSICAL DRIVERS THAT GOVERN SLITTED VALVE AND COMPONENT INTEGRITY THROUGHOUT THE PRODUCT LIFECYCLE.”
Rubber Tightness and Valve Integrity Modelling
BD is able to draw on decades of expertise in rubber component development and optimisation across its vast product portfolio, which gives it a robust understanding of the physical drivers that govern slitted valve and component integrity throughout the product lifecycle. By embedding various key inputs into physics-based simulations, BD can anticipate how the tightness of a valve evolves with time and under stress, ensuring reliable valve integrity from fill-finish through final use at point of delivery.
To assess valve tightness, BD applied Persson’s multiscale contact mechanics theory, which enables analysts to predict the sealing behaviour of a given interface, considering the materials’ mechanical properties, the roughness of both surfaces and the interface geometry.4–6 This approach enables accurate prediction of interfacial stress distributions and separations, which are critical for evaluating leakage risk (Figure 6). The valve’s slitted geometry and surrounding ribs are modelled to reflect deformation behaviour, incorporating material properties and surface roughness profiles.
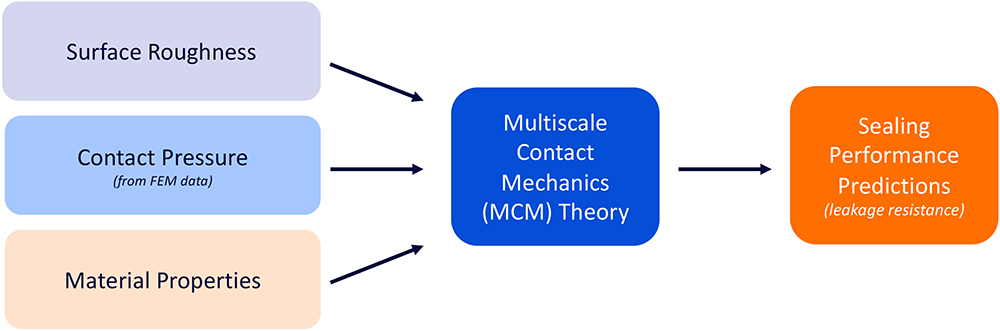
Figure 6: High-level theoretical approach leading to sealing performance predictions of a given interface.
By embedding use-case and operational parameters into the simulation, BD can anticipate how valve tightness changes over time and under mechanical or thermal stress. This includes predicting the onset of deformation and its impact on seal integrity. The modelling also supports design optimisation by identifying critical thresholds for leakage and ensuring the valve maintains reliable separation and delivery performance from manufacturing to final use.
Autoinjector Integration Modelling
With extensive experience in prefillable syringe and autoinjector system integration, BD employs a comprehensive, end-to-end modelling approach for de-risking and predicting injection performance. This modelling framework predicts key functional parameters, such as injection force and injection time, based on potential physical properties of the filled drug, including viscosity and density. The injection model developed by BD can support single- and dual-drug configurations, accounting for various secondary device parameters, enabling pharmaceutical developers to anticipate performance across a range of scenarios (Figure 7).
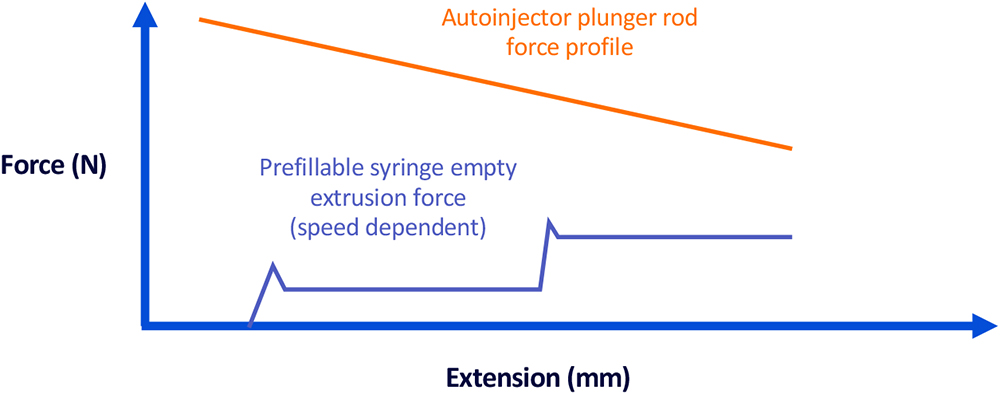
Figure 7: Graph representing the BD dual-injection syringe system force profiles in a
mock autoinjector.
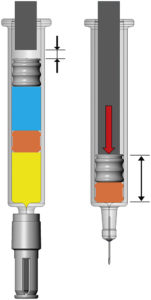
Figure 8: Simulation of BD dual-injection syringe system before and after injection.
“IMPORTANTLY, THIS MODEL IS INTERCONNECTED WITH OTHER BD SIMULATION DOMAINS, INCLUDING FEA AND FLUIDIC MODELLING, ALLOWING FOR A HOLISTIC UNDERSTANDING OF AUTOINJECTOR SYSTEM BEHAVIOUR. ”
Importantly, this model is interconnected with other BD simulation domains, including FEA and fluidic modelling, allowing for a holistic understanding of autoinjector system behaviour. This integrated approach may help to ensure that system performance can be reliably anticipated in real-world situations, enhancing confidence in device functionality and the end injection experience (Figure 8).
The result is a predictive capability that confirms the BD dual injection system maintains a similar performance profile compared with standard single-chamber prefillable syringes in an autoinjector format. This ensures streamlined integration into existing autoinjector platforms, minimising the need for design changes and supporting efficient development of combination products.
CONCLUSION
The BD dual-injection valve and syringe system was created in response to the ever-evolving pharmaceutical development pipeline, particularly in the context of biologics. As co-formulated and co-administered therapies continue to gain traction for their potential to improve efficacy, reduce the burden of injections and enhance patient outcomes, they may also introduce formulation and delivery challenges, especially when facing incongruent or reactive excipients, pH profiles or stability requirements. Dual injection, where two liquid drugs are kept physically separated until use, offers a compelling alternative to co-formulation.
The BD dual-injection valve integrates dual-chamber functionality into a standard syringe format, supporting efficient manufacturing and straightforward compatibility with existing autoinjector platforms, allowing for reliable drug delivery. By enabling pharmaceutical developers to use existing container and device systems, this innovation supports de-risked, adaptable, accelerated and scalable drug-device combination product development.
To inform and de-risk the BD dual-injection valve design, BD applied a rigorous product development pathway, using cutting-edge modelling, theories and simulation methods. Combined with extensive product expertise built on over 125 years of purpose-driven innovation, BD remains committed to delivering innovative drug delivery solutions that help its pharmaceutical partners meet the demand of today’s pipeline and tomorrow’s possibilities. To fully realise this potential, continued collaboration across the pharmaceutical ecosystem, such as with machine makers, CDMOs and autoinjector suppliers, is essential. BD invites these “partners of partners” to join in shaping the future of drug delivery and accelerating access to differentiated therapies for patients worldwide.
*The BD dual-injection valve and syringe system is a product in development. Some statements made are forward-looking and are subject to a variety of risks and uncertainties.
ACKNOWLEDGEMENTS
The authors would like to thank Simon Rempfer, Julien Singer and Stephane Huot for their contributions to this article.
REFERENCES
- Chauhan V M et al, “Advancements in the co-formulation of biologic therapeutics”. J Control Release, 2020, Vol 327, pp 397–405.
- Mueller C, Altenburger U, Mohl S, “Challenges for the pharmaceutical technical development of protein coformulations”. J Pharm Pharmacol, 2018, Vol 70(5), pp 666–674.
- Krieg D, Winter G, Svilenov H L, “It is never too late for a cocktail: Development and analytical characterization of fixed-dose antibody combinations”. J Pharm Sci, 2022, Vol 111(8), pp 2149–2157.
- Rodriguez N, Tiwari A, Persson B N J, “Air leakage in seals with application to syringes. Appl Surf Sci Adv, 2022, Vol 8, art 100222.
- Yang C, Persson B N J, “Contact mechanics: Contact area and interfacial separation from small contact to full contact”. J Phys: Condens Matter, 2008, Vol 20(21), art 215214.
- Lorenz B, Persson B N J, “Leak rate of seals: Effective- medium theory and comparison with experiment”. Eur Phys J E, 2010, Vol 31, pp 159–1

