To Issue 177
Citation: Neumeier M, “Advancing Lyophilised Drug Development in Dual-Chamber Systems”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 62–65.
Dr Markus Neumeier details the lyophilisation process and the range of elements that need to be examined for a dual-chamber system undergoing freeze-drying, while also laying out the quality control processes to conduct when transitioning from the laboratory to industrial-scale operations.
The injectable drug market is continuing to evolve in response to the growing demand from pharmaceutical and biotech companies for innovative delivery systems that improve patient convenience and safety. Prefilled systems offer a streamlined, user-friendly alternative that provides advantages in terms of safety, dosing accuracy and lifecycle management.
Dual-chamber systems provide a packaging and delivery system that can offer significant added value to parenteral drug products. These systems contain both the lyophilised drug and its diluent in a single device, separated by a middle stopper. This innovative system is an alternative to traditional vial-and-syringe combinations and greatly simplifies the administration process, reduces the risk of contamination and effectively minimises drug overage (Figure 1). However, the lifecycle of a drug product, from a lyophilised vial to a dual-chamber system, faces a variety of technical and regulatory challenges.
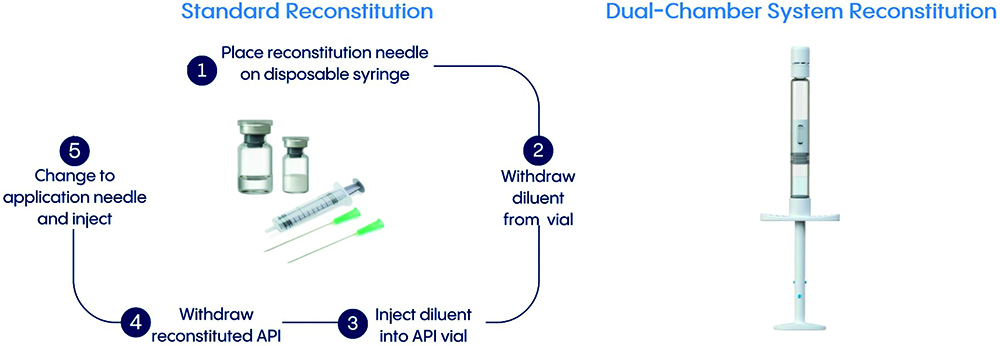
Figure 1: Comparison of traditional vial syringe versus dual-chamber system reconstitution (example shown is Vetter Lyo-Ject® syringe).
PROCESS DEVELOPMENT FOR LYOPHILISED DRUG PRODUCTS
The successful development of a lyophilised product in a dual-chamber system requires an in-depth understanding of the formulation’s properties, the packaging materials and the parameters of the manufacturing process and equipment. A robust lyophilisation cycle is founded on the thermal characterisation of the unique API formulation. Once these elements have been identified, they undergo a holistic, small-scale development process to create a strong package. Completion of this process leads to a successful qualification of a lyophilised product in a dual-chamber system.
The lyophilisation cycle is usually divided into three phases: the freezing phase, primary drying and secondary drying. Each phase requires specific input parameters, such as shelf temperature and chamber pressure, which must be correlated with output parameters, such as product temperature and sublimation rate. Using a “Design of Experiments” approach involving different temperature and pressure combinations can help to establish a product specific design space and specification limits.
Packaging Materials
The transition from a lyophilised vial to a dual-chamber system requires careful evaluation of the packaging components. A typical dual-chamber syringe consists of a siliconised glass barrel, middle and end stoppers and a closure part. Compared with vials, the glass barrel for a dual-chamber system is longer and has a smaller inner diameter, affecting heat transfer during lyophilisation.
“THE UNIQUE GEOMETRY AND SILICONISATION OF THE PACKAGING COMPONENTS INFLUENCE THE FREEZE-DRYING PROCESS.”
The unique geometry and siliconisation of the packaging components influence the freeze-drying process. The design of the dual-chamber glass barrels results in a sublimation process that is separate from direct shelf contact. Unlike vials, dual-chamber systems require magazine-based processing to maintain an upright position throughout the manufacturing process. Additionally, the sealing must occur under atmospheric pressure as opposed to under partial vacuum to prevent stopper movement.
Thermal Product Properties
Compared with lyophilisation in a vial with direct contact to the freeze dryer shelves, the drying process in dual-chamber systems is governed by different driving forces – primarily by convection and radiation rather than direct heat conduction from the shelves. These altered heat transfer dynamics necessitate closer scrutiny of sublimation behaviour. Key parameters such as fill volume, cake height and mass concentration directly affect product resistance and drying performance.
Therefore, it is essential to monitor product temperature and drying time, as these are linked to output parameters such as cake appearance and residual moisture levels. These factors must be evaluated alongside critical quality attributes during drug product stability studies to validate the lyophilisation cycle.
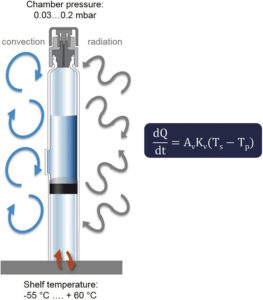
Figure 2: Heat transfer mechanisms during freeze drying in a dual-chamber system.
Heat Transfer Mechanisms in Freeze Drying
During lyophilisation, heat transfer in a dual-chamber system occurs via conduction, convection and radiation. The sum of all three mechanisms determines the overall heat transfer rate (dQ/dt), which can be derived using the formula shown in Figure 2. It uses the following variables: (Ts – Tp) for the difference between the shelf temperature and the product temperature; Av for the cross-sectional area of the glass barrel; and Kv for the heat transfer coefficient. In a dual-chamber system, convection dominates during the freezing phase due to atmospheric pressure, while radiation becomes the primary mechanism during primary and secondary drying under vacuum conditions. This is a major difference compared with lyophilised vials.
As the product is not in direct contact with the shelf, its temperature is only indirectly influenced by the shelf temperature. This makes drug products in dual-chamber systems more susceptible to edge effects, regardless of formulation or fill volume. Understanding these dynamics is crucial for designing a robust lyophilisation cycle.
Sublimation Rate Considerations
The sublimation rate, a key determinant of drying efficiency, is governed by the total resistance within the system, including:
- Resistance from the container
- Resistance from the drug product
- Resistance from the freeze dryer itself.
The dimensions of the glass barrel and the design of the closure part significantly influence the resistance of the container. The resistance of the drug product depends on the drying surface, fill height, formulation and maximum mass concentration.
The driving force behind the sublimation process in a lyophiliser is the gradient in water vapour pressure between the sublimation front and the chamber pressure. As the fundamental principle of freeze drying, water vapour is drawn into the condenser to create a sublimation flow. A detailed look inside the freeze dryer shows a heterogeneous distribution of flow, with water vapour pressure having a strong effect on sublimation of the drug product. Although the opening of the glass barrel can restrict vapour flow into the lyophilisation chamber, the main factor influencing sublimation is still the vapour pressure from the condensed phase of the drug product.
Process and Equipment Parameters
A thorough understanding of the properties of packaging and equipment, heat transfer and sublimation behaviour are essential for developing a lyophilisation cycle. This theoretical knowledge must be combined with the product-specific properties of the formulation. Determining the glass transition temperature of amorphous formulations and the eutectic temperature of crystalline formulations provides relevant parameters with which to evaluate the design space for lyophilisation cycle development (Figure 3). Thermal analysis techniques, differential scanning calorimetry and freeze-drying microscopy help to identify critical formulation parameters.
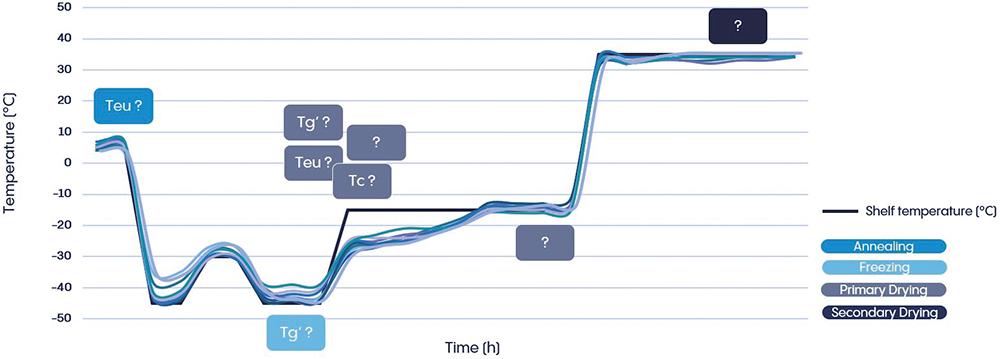
Figure 3: Illustration of freeze-drying cycle development – considering the relevant parameters for each phase during lyophilisation cycle design (Teu – Eutectic temperature; Tg’ – Glass transition temperature; Tc – Collapse temperature).
“THESE TARGETED EXPERIMENTS OFFER VALUABLE INSIGHTS INTO BOTH THE FORMULATION AND THE INTENDED MANUFACTURING PROCESS, HELPING TO ESTABLISH A SOLID FOUNDATION FOR FURTHER DEVELOPMENT.”
Small Scale Development
Lab-scale studies play a crucial role in thoroughly characterising each phase of the lyophilisation cycle. These targeted experiments offer valuable insights into both the formulation and the intended manufacturing process, helping to establish a solid foundation for further development. As they require only minimal quantities of the API, they are not only cost-effective but also highly adaptable to changing conditions. Their flexibility allows for rapid variations of process parameters and facilitates the early identification of potential failure points. This, in turn, supports the design and refinement of product-specific lyophilisation cycles tailored to the unique properties of each formulation.
Once the lyophilisation cycle has been successfully optimised at the laboratory scale, established scale-up factors can be applied to transfer the process to commercial manufacturing environments (Figure 4). This allows for consistency and reproducibility across production batches, helping to maintain product quality and regulatory compliance.
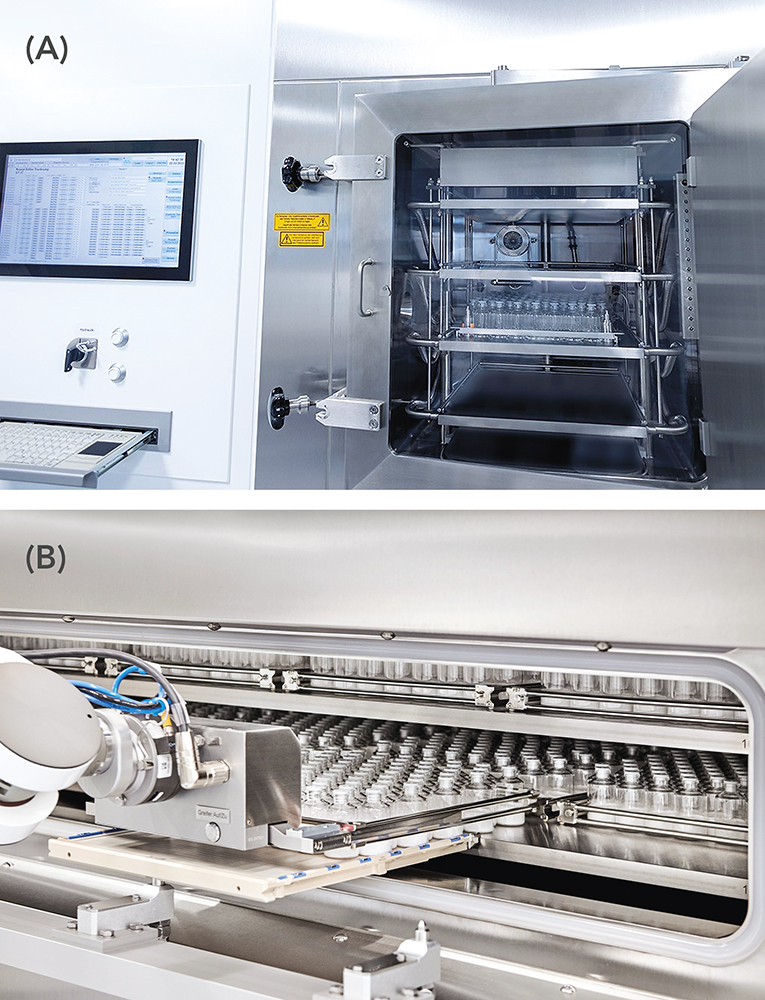
Figure 4: Scale-up – (A) from lab lyophiliser for small-scale studies to (B) automated, commercially operated lyophiliser.
Qualification of Lyophilised Products
Lab-scale development results form the foundation of a risk-based control strategy for commercial production, and engineering runs on the intended commercial filling line and freeze dryer serve as the starting point for the overall validation strategy of the drug product freeze-drying cycle. The intended strategy is also supported by a bracketing approach, which covers minimum and maximum loads, the use of multiple lyophilisers and varying fill volumes.
The success of the lyophilisation cycle qualification depends on thorough equipment qualification. Spatial uniformity of product temperature and critical quality attributes are assessed using a star-pattern mapping technique. These data identify hot and cold spots, providing essential information for understanding the specific heat transfer coefficient of the shelf and freezer dryer, which enables the impact of these elements on the critical quality attributes of the drug product to be evaluated further on a more case-specific basis. Authorities often require this star mapping to identify the worst positions of the freeze dryer.
Process analytical technology systems are used to monitor the product temperature in relation to the shelf temperature at each individual position. Samples taken from distinct positions confirm the consistency of critical quality attributes, thus validating the lab-scale findings and supporting a reproducible manufacturing process.
Management of Lyophilised Drug Product Lifecycle
Dual-chamber systems offer clear advantages over traditional vial-and-syringe combinations and represent a further step in lifecycle management. However, developing a lyophilised drug product in a dual-chamber system is challenging and requires comprehensive expertise and know-how.
Risk-based development within a defined design space demonstrates that the duration of both primary and secondary drying phases is dependent on product temperature, as confirmed through the design of experiments. This science-driven approach results in a robust data package. By integrating thermal analysis, small-scale experimentation and robust qualification strategies, pharmaceutical manufacturers can design a lyophilisation cycle that meets the critical quality attributes and regulatory expectations of drug products.

