To Issue 177
Sugalski E, “Rethinking Dual-Chamber Syringes: A Lateral Approach to Faster, Safer Reconstitution”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 79–83.
Eric Sugalski examines the limitations of current concentric dual-chamber syringe designs and explores a novel, lateral-chamber approach from Ampulis – the Ampulis Reconstitution Syringe.
Reconstituted drugs hold enormous promise – from improving stability and extending shelf life to enabling life-saving therapies. But delivering these therapies to patients efficiently, accurately and safely is far from straightforward. Dual-chamber syringes (DCSs) have emerged as a seemingly elegant solution – a single device that stores lyophilised drug and diluent separately, then enables quick reconstitution at the point of use. On paper, DCS technology offers convenience, faster preparation and reduced risk of contamination.
In practice, however, the story is more complicated. Despite years of availability, DCS devices have yet to see broad market adoption. Beneath the surface lie persistent usability, manufacturing and cost challenges that limit their real-world uptake. Variability in activation forces, awkward plunger strokes, intricate tooling requirements and complex fill-finish processes all combine to create friction – both literal and figurative – in their path to widespread use.
By rethinking the basic geometry and using simpler, more reliable manufacturing methods, Ampulis’s novel lateral-chamber approach, the Ampulis Reconstitution Syringe (ARS), holds promise to solve the very problems that have long hindered DCS adoption – and to open the door for faster, safer and more user-friendly reconstitution both in clinical and at-home settings.
THE DCS DILEMMA
While the concept of DCS technology is sound, the execution, particularly with today’s dominant concentric designs, has exposed practical and economic hurdles, which are difficult to overcome. What appears at first glance to be a simple adaptation of a standard prefilled syringe actually introduces a cascade of engineering, usability and manufacturing complexities. These challenges do not just impact the user experience; they ripple through the entire product lifecycle, from device design and production to fill-finish operations and final market viability.
Understanding why adoption has stalled requires a closer look at the most common pain points with current DCS systems, and why these issues matter for both patient safety and commercial success.
There are numerous factors involved, but we will consider some of the known challenges with existing DCS devices that may be limiting market uptake.
Variable Activation Forces
Most DCS devices are designed in concentric form, wherein the lyophilised formulation (lyo) and diluent chambers are coaxial. While this design appears simple and familiar to existing prefilled syringes, some significant differences and challenges require consideration. One of these challenges relates to the difference in frictional resistance during the displacement of multiple stoppers within the DCS. If a user applies a constant force at the end of the plunger rod, the displacement velocity will vary depending on the position of the stoppers and the pressurisation of air pockets within the two chambers. The velocity will also differ significantly between break-loose, diluent transfer and the extrusion stages. This rapid change in frictional resistance not only creates confusion and fatigue for users but can also result in incomplete diluent transfer that affects dose accuracy.
Long Plunger Stroke
Another usability challenge associated with the concentric DCS configuration is the necessity for the lyo compartment to accommodate the full volume of diluent. Additionally, both lyo and diluent chambers require air pockets to facilitate diluent transfer. These air pockets significantly increase the travel required of the plunger rod, as the stroke needs to accommodate not only the dispensing of the reconstituted formulation but also the transfer of the diluent into the lyo compartment and the compression of multiple air pockets. For many users, this requires a two-handed operation, which is much less controlled than a precision grip with a single hand. Compounded with the variable activation force issue already mentioned, this presents further human factors and dose-accuracy challenges.
Complex and Costly Manufacturing
Existing DCS devices require bypasses and/or valves to transfer diluent into lyophilised formulation chambers. These intricate features require complex and expensive tooling with tightly controlled processing requirements to produce reliable components. These manufacturing complexities translate into high non-recurring engineering and unit costs for DCS devices.
A NEW TWIST ON DCSs
Rather than a concentric configuration, consider one where the chambers are positioned laterally to one another. This is the basis for the ARS. In between these two lateral chambers is a small passage that is used for diluent transfer. Additionally, a simple one-way valve is used to prevent backflow. Figure 1 illustrates two versions of ARS.
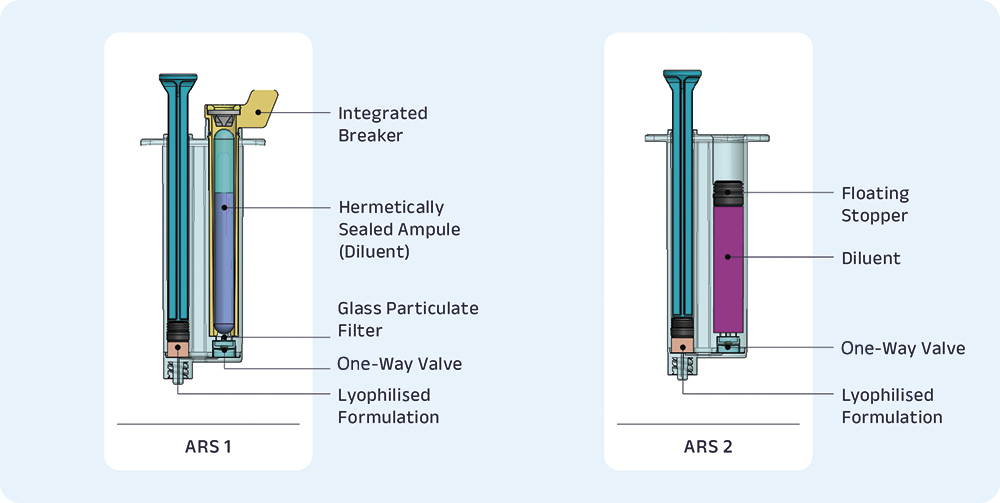
Figure 1: Ampulis ARS1 and ARS2 platforms.
“THE LATERAL CONFIGURATION OF ARS MINIMISES FRICTIONAL VARIATION, IMPROVES USABILITY AND INCREASES DOSE RELIABILITY.”
In both configurations, the DCS problems previously mentioned are resolved.
- Following stopper break-loose, the glide force is constant. Unlike existing DCS devices, there are no bypass sections, momentarily actuated valves or multistopper displacements that cause glide force variability. The lateral configuration of ARS minimises frictional variation, improves usability and increases dose reliability.
- Plunger height is near-standard and stored in its collapsed position. The ARS design avoids the air pockets in existing DCS devices that are required for diluent transfer. As a result, the plunger stroke is nearly identical to that of standard prefilled syringes, even for larger fluid volumes. Furthermore, since the plunger rod is stored in its collapsed state, the packaged product height is considerably shorter than concentric DCS devices, which can present advantages for shipping and inventory management.
- ARSs use conventional drug-compliant materials and manufacturing processes. They do not need the intricate valves, cannulas and bypass features that require complex tooling and tightly toleranced components.
ARS1 – DILUENT IN AMPOULE
This first configuration of ARSs aims to simplify the fill-finish process by eliminating the subsequent filling steps following lyophilisation. With the ARS assembly in final form, the lyophilisation process can occur, immediately followed by packaging. Additionally, the ARS1 configuration ensures that there is no possibility for premature mixing of diluent and lyophilised formulation. Some of the key features of the ARS1 configuration include:
Hermetically Sealed Ampoule
The diluent is stored within a hermetically sealed ampoule. This provides intrinsic container closure integrity and minimises risks related to extractables and leachables due to the all-glass contact surface with the diluent. Furthermore, this ampoule ensures that there is no possibility for premature mixing of lyophilised formulation and diluent.
Integrated Breaker
A simple rotating component is integrated into the device to easily fracture the ampoule, which releases the diluent into the ampoule chamber. This integrated breaker is an injection moulded component that creates a force concentration on the ampoule at a specific location for reliable fracturing.
Embedded Glass Particulate Filter and Valve
A filter is integrated into ARS1 to eliminate all-glass particulate while transferring the diluent into the lyophilised chamber. Additionally, a one-way valve is embedded to prevent backflow of reconstituted drug into the ampoule chamber after the diluent has been transferred.
ARS2 – NO AMPOULE
The second configuration of ARS excludes the ampoule and the integrated breaker. This ARS2 configuration can accommodate larger drug volumes and has similar manufacturing and fill-finish processes to ARS1. Some of the unique aspects of ARS2 include:
Floating Stopper
On the diluent side of the ARS2 is a cartridge-style stopper that sustains the the hydrostatic pressure maintained between the lyo and diluent chambers. As the user displaces the plunger rod, this pressure is translated through the passage and into the diluent chamber, resulting in consistent and accurate displacement of the floating stopper.
Embedded One-Way Valve
Similar to ARS1, ARS2 also includes a one-way valve that prevents backflow of the reconstituted formulation into the diluent chamber. However, ARS2 does not require the embedded glass particulate filter because it does not contain a glass ampoule.
Autoinjector Applications
This version of ARS2 is well-suited to autoinjector applications, which are currently under development at Ampulis.
STEPS OF OPERATION
The steps involved in operating the ARS have been designed with the user in mind. Each of these steps are familiar actuations that are common in standard drug delivery processes. Figures 2–4 illustrate the primary steps involved in operating ARS1.
Step 1: Rotate Breaker
As illustrated in Figure 2, the first user step is to rotate a tab at the top of the device, which causes pressure to be applied at the lower end of the ampoule, resulting in the ampoule fracturing and releasing the diluent into the ampoule chamber (for ARS2, this step is avoided, as there is no ampoule).
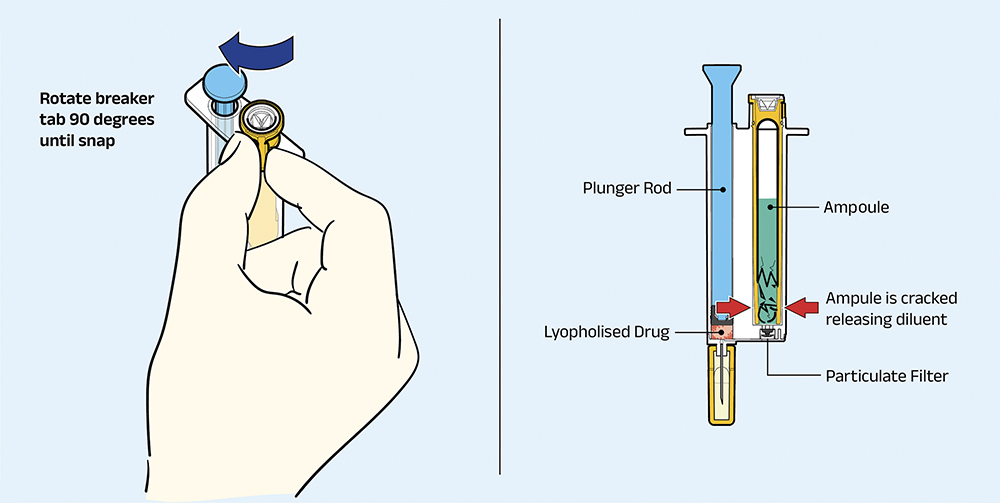
Figure 2: Rotate breaker tab to access diluent.
Step 2: Draw Syringe Upwards
As shown in Figure 3, the user then draws the syringe upwards. This causes the diluent to flow from the ampoule chamber into the lyo chamber. This transfer process forces the diluent through the embedded filter, which eliminates any glass particulate. The diluent proceeds through a one-way valve, which prevents it from backflowing into the ampoule chamber (this user step is identical for ARS2).
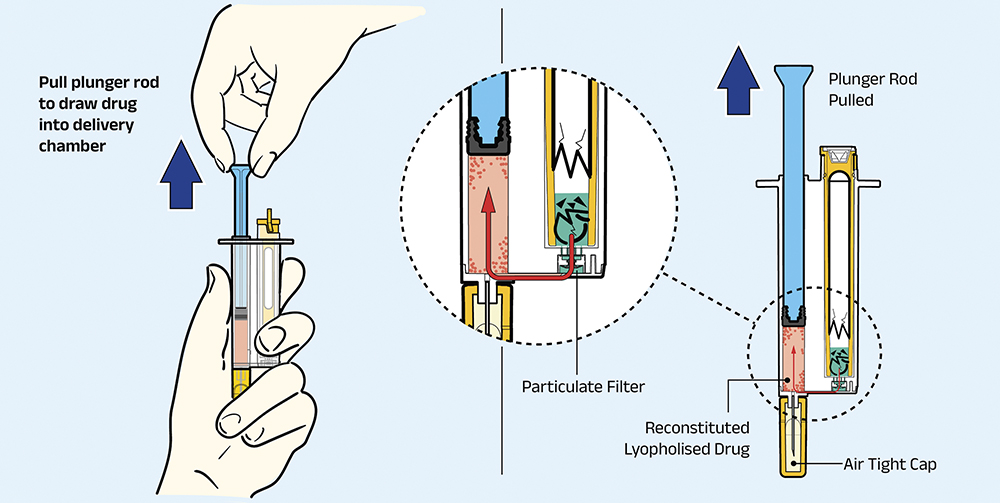
Figure 3: Pull plunger to combine diluent with lyophilised therapeutic.
Step 3: Prime and Inject
As illustrated in Figure 4, the last step in operating ARS1 is to prime the reconstituted therapeutic and inject – an identical step to that used in a standard prefilled syringe (this step is identical for ARS2).
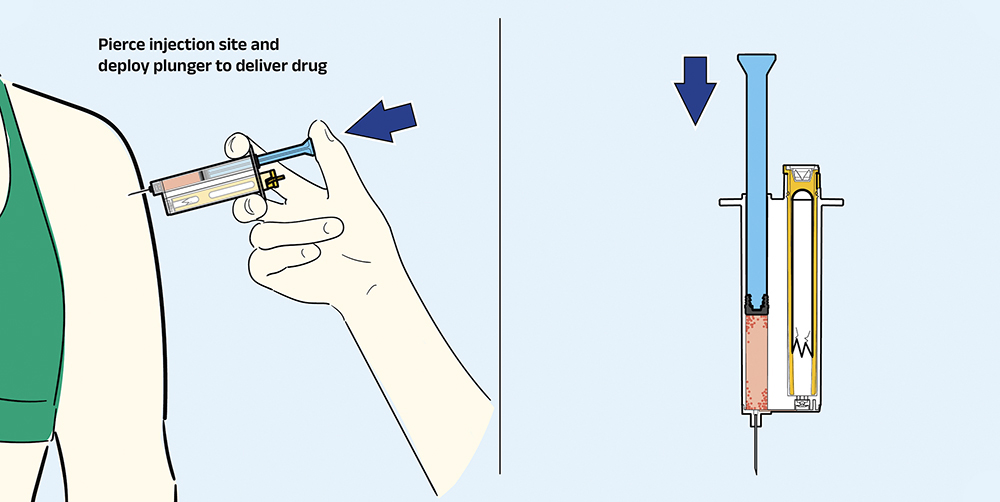
Figure 4: Prime and inject therapeutic.
CONFIGURATION OPTIONS
While ARS configurations are already optimised for usability, there are often unique elements of therapeutics and use cases that must be considered. For these unique applications, ARSs can be customised across several parameters. Some of these opportunities for customisation include:
Multiple Dose Volumes
ARSs can be produced in various dose volumes. Graduations and dose marks can also be customised to specific formulation requirements. Figure 5 illustrates three potential dose volume options for ARS1. ARS2 can achieve larger volumes (up to 20 mL).

Figure 5: ARS 1.0 mL (left), ARS 2.25 mL (centre), ARS 3.0 mL (right).
Needle Options
ARSs can be produced with staked needles in various gauges and depths for subcutaneous and intramuscular administration modes. Additionally, ARSs can be produced with a luer connection for intravenous administration or to accommodate user-applied needle subassemblies.
Safety Cover Options
In addition to the needle options, ARSs can be configured with an integrated safety cover to accommodate OSHA requirements.
Branding and Labelling Options
Colours, logos and other labelling can be customised to meet the customer’s brand requirements while providing users with sufficient information for safe and effective use.
COLLABORATING FOR USABILITY, COST SAVINGS AND FASTER MARKET ENTRY
For years, DCSs have promised to streamline reconstitution for lyophilised drugs, offering faster preparation, reduced contamination risk and a simpler patient experience. Yet widespread adoption stalled, primarily due to the limitations of concentric DCS designs. These devices often suffer from inconsistent activation forces, long and awkward plunger strokes, intricate manufacturing requirements, and expensive, highly specialised fill-finish processes. The result: higher per-unit costs, complex supply chains and usability challenges that can affect both patient safety and market acceptance.
The ARS rethinks the DCS from the ground up with a lateral-chamber design that eliminates bypasses, multistopper displacement and air pockets. This results in constant glide force, standard plunger stroke, and simpler, more reliable manufacturing and fill-finish process, reducing capital investment and unit cost. For pharmaceutical partners, ARS offers not only significant cost savings and manufacturing efficiency but also improved usability, fewer human factors risks and a streamlined regulatory pathway. In short, ARS makes the commercial case for reconstituted therapies stronger than ever, accelerating development, lowering risk and improving patient outcomes.
BIBLIOGRAPHY
- Babaee S et al, “A modeling framework for spring-driven autoinjectors with dual-chamber cartridges”. Drug Deliv and Transl Res, 2025.
- Werk T et al, “Technology, Applications, and Process Challenges of Dual Chamber Systems”. J Pharm Sci, 2016, Vol 105 (1), pp 4–9.

