To Issue 179
Citation: Dao T, “Closing the Loop: Designing the Future of Sustainable Drug Delivery Devices”. ONdrugDelivery, Issue 179 (Oct/Nov 2025), pp 8–12.
Thuysi Dao outlines a two-pronged approach to improving the sustainability profile of drug delivery devices, combining sustainable device design with real-world take-back and recycling initiatives, and explores how Ypsomed’s new YpsoLoop autoinjector platform embodies this vision.
As the pharmaceutical industry accelerates its drive towards net zero, the environmental impact of drug delivery devices is coming sharply into focus. Scope 3 emissions dominate company footprints and end-of-life processing represents a significant proportion of a device’s carbon dioxide (CO2) emissions. This makes it essential to rethink device design and end-of-life processes.
CARING FOR PATIENTS, IMPACTING THE PLANET
With the purpose of protecting and enhancing human health, the healthcare sector plays a vital role in society. However, it is also a substantial contributor to the climate crisis, which is increasingly recognised as one of the greatest health threats of the modern era.1 This paradox places the industry in a unique position – while delivering essential care, it must also confront and reduce the environmental impact of its own activities. The healthcare sector is responsible for 4.4% of net global greenhouse gas emissions – a footprint so large that it would rank as the fifth biggest climate polluting country. The pharmaceutical industry is a major contributor to this, and without decisive change, the sector’s carbon impact is projected to triple by 2050.2
“RECENT DISCLOSURES FROM MAJOR PHARMACEUTICAL COMPANIES CONSISTENTLY SHOW THAT SCOPE 3 EMISSIONS – THOSE GENERATED INDIRECTLY ACROSS THE VALUE CHAIN – ACCOUNT FOR BETWEEN 77% AND 98% OF THEIR TOTAL EMISSIONS, FAR EXCEEDING SCOPES 1 AND 2.”
Many global pharmaceutical companies have committed to ambitious, science-based targets verified by the Science Based Target initiative (London, UK). These aim to significantly reduce Scope 1 and 2 emissions by 2030 and to reach net zero emissions across all scopes by 2050 at the latest.3 Such commitments are vital in aligning the industry’s core mission of safeguarding health with the equally urgent need to protect the planet.
Recent disclosures from major pharmaceutical companies consistently show that Scope 3 emissions – those generated indirectly across the value chain – account for between 77% and 98% of their total emissions, far exceeding Scopes 1 and 2 (Figure 1). Within Scope 3, the lion’s share of emissions clearly lies within the categories of purchased goods and services (41–48% in biotech, pharma and medtech) as well as the use of sold goods (29%).4
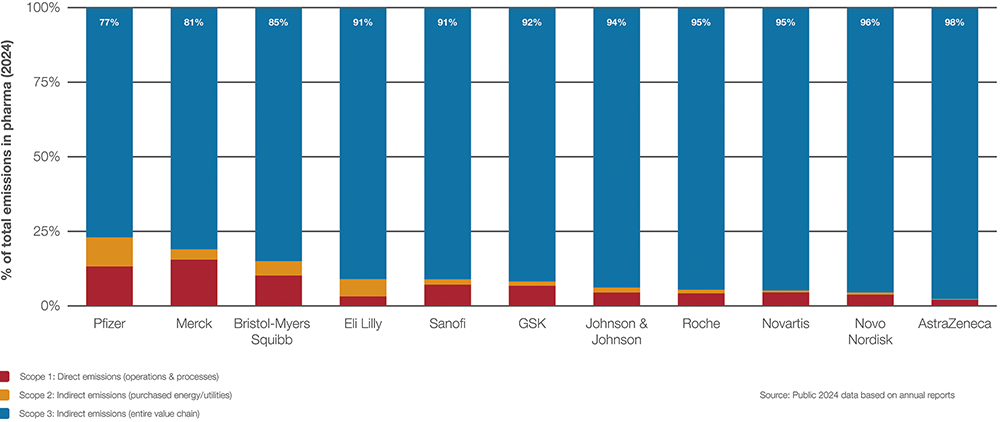
Figure 1: Breakdown of Scope 1 to 3 emissions in leading pharmaceutical companies.
THE DEVICE LENS: A TANGIBLE LEVER FOR CO2 REDUCTION
At the pharmaceutical product level, devices represent a significant source of CO2 emissions and, consequently, a major opportunity for Scope 3 reduction as pharma works with suppliers to meet ambitious climate targets. The environmental footprint of injection devices extends from the extraction of raw materials to their final disposal. A lifecycle assessment of a disposable 1 mL autoinjector shows that raw materials (40%) and end-of-life disposal (20%) are the largest individual contributors to emissions, followed by packaging (15%). The remainder results from manufacturing processes, transportation and waste handling (Figure 2).5
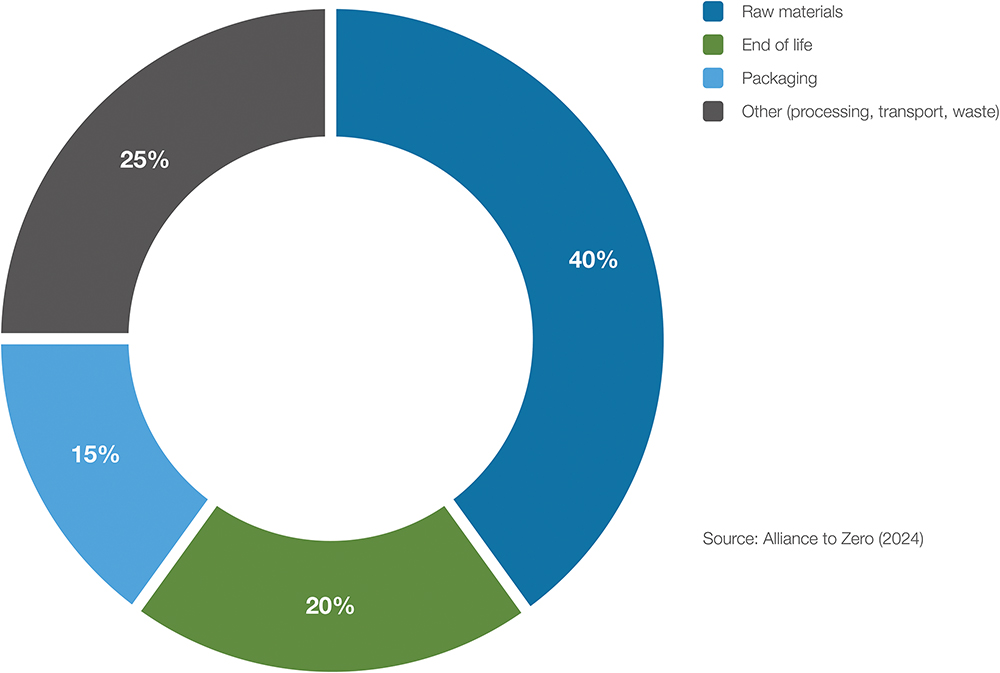
Figure 2: CO2 emissions breakdown of a disposable 1 mL autoinjector.
Reducing this footprint requires action to deal with the major contributing factors. For raw materials, this includes, among other measures, selecting lower-impact alternatives, sourcing responsibly and minimising material use where possible. For end-of-life, the current approach is almost entirely limited to high-temperature incineration or landfill disposal, with recycling not yet a significantly explored pathway. This “hidden cost of disposability” highlights the need for sustainable innovation that addresses not only material choice but also the design of the devices for recycling and disassembly, ensuring compatibility with emerging recovery systems. It is also necessary to advance these systems themselves to enable them to handle medical devices and capture resources at scale.
TWO PERSPECTIVES FOR IMPACT: WHY DEVICE DESIGN AND END-OF-LIFE MATTERS
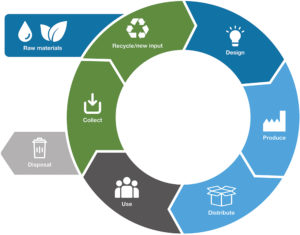
Figure 3: Circularity requires sustainable product design and end-of-life recovery solutions.
Addressing these drivers requires action from two complementary angles:
- Circular Design: Designing devices with sustainability in mind from the outset
- Closing the Loop: Building the systems needed to collect, recycle and reintegrate device materials.
One without the other leaves potential circularity untapped. To move from open-loop recycling towards true closed-loop systems, sustainable device design and take-back initiatives must evolve hand in hand (Figure 3).
“DESIGN DECISIONS MADE DURING THE INITIAL CONCEPT STAGE CAN DETERMINE UP TO 80% OF A PRODUCT’S ENVIRONMENTAL FOOTPRINT.”
Perspective 1: Designing for Circularity
Circularity starts long before a device is produced – it begins at the drawing board. Design decisions made during the initial concept stage can determine up to 80% of a product’s environmental footprint.6 Therefore, integrating sustainability principles from the outset involves adopting the “R-strategies” as a design philosophy rather than an afterthought:
- Reduce: The first and most impactful step involves selecting materials with the lowest possible environmental footprint, minimising the number of different polymers used and reducing the overall material volume. This directly lowers embedded carbon and resource use.
- Reuse: The second step focuses on extending a product’s lifecycle, allowing it to remain in service for longer, thereby reducing the need for new materials and additional manufacturing.
- Recycling: The third and final step closes the loop by ensuring that materials can be recovered and reprocessed. However, this is only viable when the product is designed for disassembly and material separation, as well as being made compatible with available recycling infrastructure.
Ypsomed has embedded these principles into its eco-design guidelines, translating them into concrete ambitions. These include reducing weight and material, minimising material types, prioritising recyclable mono-materials, incorporating certified bio-based feedstocks where possible and ensuring that products are designed for disassembly from the outset. By integrating these strategies into the concept phase, Ypsomed aims to maximise circularity, ensuring that, when recovery systems are ready, the devices will be too.
Perspective 2: Closing the Loop
Take-back schemes are gaining traction in the pharmaceutical industry. Programmes such as Johnson & Johnson’s SafeReturns and Novo Nordisk’s ReMed and returpen demonstrate that collection is possible, albeit on a modest scale. Beyond the environmental benefits, many companies across various industries also see potential economic value – over 70% of those engaging in circularity initiatives expect a positive financial impact by 2027/2028.7
However, moving from today’s practices to full circularity is a multistep process. Due to regulatory limitations, devices are still produced using 100% virgin-grade plastics. Some progress is being made through recovering production waste, wherein clean moulding scrap is reintroduced or recycled into non-medical applications, and open-loop recycling, in which post-user plastics are recovered and downcycled into non-medical components. Nevertheless, the ultimate goal for many is closed-loop recycling, meaning the recovery of used devices and decontaminating and reprocessing them into medical-grade components. Most projects currently stop at open-loop solutions, so the transition to closed-loop is both a challenge of innovation and an opportunity for leadership.
However, scaling up these circularity initiatives for self-injection devices presents several challenges:
- Technical: Devices are complex assemblies of mixed materials that are often contaminated with biological residue. Current recycling technologies struggle to separate these components safely, reliably and efficiently.
- Regulatory: Used injection devices are classified as medical waste, which restricts transport and processing. Regulations around the use of recycled materials in medical devices are still evolving, creating uncertainty for manufacturers.
- Systemic/Infrastructure: Collection systems for medical devices are fragmented or absent in most markets. Building them requires investment, alignment between stakeholders and, often, pre-competitive collaboration across the industry.
- Commercial and Scale: Many pilot programmes remain geographically limited and achieve relatively low return rates. Uncertainty regarding financial viability, actual CO2 reduction potential at scale and the industry’s long-term direction may hinder investment and commitment.
While these challenges are significant, they are not insurmountable. Addressing them requires long-term planning, clear policy direction and partnerships across the healthcare ecosystem. This collaborative environment provides an opportunity to develop, implement and trial value-added solutions that transcend compliance, establishing companies as pioneers in sustainable innovation and laying the groundwork for scalable, industry-wide systems.
“YPSOLOOP, YPSOMED’S NEW PREFILLED AUTOINJECTOR PLATFORM, IS ONE EXAMPLE OF THIS APPROACH, EMBODYING BOTH SIDES OF THE CIRCULAR INNOVATION LOOP.”
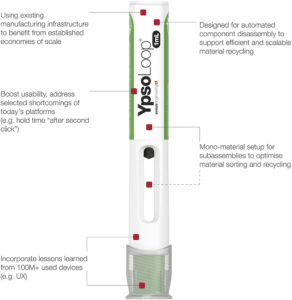
Figure 4: Ypsomed’s new YpsoLoop platform.
YPSOLOOP: RETHINKING PLATFORM DESIGN FOR SCALABLE DISASSEMBLY AND EFFICIENT MATERIAL RECOVERY
Solutions that work in both dimensions are required to bring together the principles of circular design and the need for real-world recovery systems. YpsoLoop, Ypsomed’s new prefilled autoinjector platform, is one example of this approach, embodying both sides of the circular innovation loop (Figure 4).
Developed under eco-design principles embedded in Ypsomed’s ISO 14001- certified processes, YpsoLoop is able to achieve a significantly lower material CO2 footprint. It uses bio-based plastics and is built around two mono-material subassemblies that allow for easy material separation. Its design for disassembly architecture is the first of its kind to enable scalable, automated component separation, making cost-efficient material recovery in take-back schemes a realistic possibility, paving the way for closed-loop recycling.
Positioned to set a new benchmark for sustainable drug delivery platforms, Ypsomed’s ambition goes beyond offering a sustainable product. The company’s goal is to deliver a solution that connects sustainable device innovation with real-world recovery systems and accelerates circularity across pharma. Ypsomed is driving this forward by working with partners and consortia to proactively embed YpsoLoop into take-back programmes, while also exploring new pathways and concrete solutions that make circularity more scalable. The aim is to lower the hurdles for pharmaceutical clients to adopt circularity and help move the industry from today’s linear status quo towards open- and, ultimately, closed-loop recycling, ensuring that device design and recovery infrastructure advance together (Figure 5).
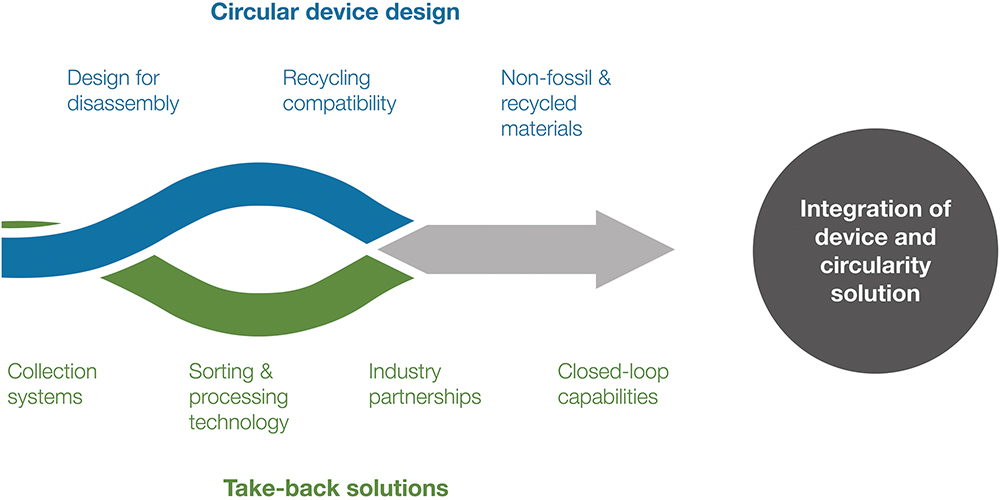
Figure 5: Joint progress in circular device design and take-back solutions, which are needed to advance circularity.
A CALL TO ACTION FOR THE INDUSTRY
Circularity in drug delivery devices is within reach, but only if the industry is prepared to make sustainability a primary requirement rather than a secondary consideration. It is, above all, the internal decisions around device design and architecture that will determine how far the industry progresses. The industry must consider how willing it is to reduce complexity in favour of recyclability, since this is an area it can influence directly. This requires a willingness to accept recycled content where it is safe and compliant, to prioritise recyclability over colour customisation and to collaborate pre-competitively on shared material standards and infrastructure. Investing in collection and recycling systems alongside product innovation is also essential to ensure that sustainability gains can be realised at scale.
The question is whether the industry is ready to radically rethink its approach to devices to meet sustainability ambitions. As Albert Einstein observed, one cannot solve their problems with the same thinking used when creating them. For the pharmaceutical and medtech sectors, this means challenging long-held assumptions and approaches about design, customisation, manufacturing and end-of-life management. By keeping valuable materials in circulation and reducing the environmental impact of drug delivery, the industry can continue to provide life-changing therapies while safeguarding the health of the planet. For Ypsomed, this ambition is set and circularity is already a central principle of its innovation, guiding the choices it makes right now.
REFERENCES
- Ghebreyesus TA, Al Jaber A, Kerry V, “We must fight one of the world’s biggest health threats: climate change”. World Health Organization, Nov 2023.
- “Health Care’s Climate Footprint”. Health Care Without Harm, Sep 2019.
- “Decarbonisation in Pharma and Healthcare: State of the Sector 2025”. SLR Consulting, Aug 2025.
- “The Carbon Impact of Biotech & Pharma”. Report, My Green Lab, 2024.
- “Net Zero Requires Circularity”. Alliance to Zero, Jul 2024.
- “An introduction to circular design”. Ellen MacArthur Foundation, Jun 2022.
- “Circular Transformation of Industries: Unlocking Economic Value”. World Economic Forum, Jan 2025.
Previous article
A BREATH OF FRESH AIR: PHARMA’S PUSH FOR SUSTAINABLE pMDIsNext article
EXPANDING OPPORTUNITIES FOR INHALED DRUG DELIVERY
