To Issue 180
Citation: Clayton J, “Dissolution Testing for Orally Inhaled Products: Confronting the Challenges, Realising the Potential.” ONdrugDelivery, Issue 180 (Nov 2025), pp 79–83.
As dissolution testing rises up the agenda for orally inhaled product developers, Jamie Clayton explores drivers behind its adoption and key considerations for developing a robust and relevant method.
Dissolution testing has long been a topic of interest for the inhaled drug product community. This is logical given that, following deposition in the lung, dissolution is the first step towards absorption and therapeutic effect for both locally and systemically acting drugs.
“TODAY’S OIP DEVELOPERS FIND THEMSELVES WITH A GROWING BODY OF BACKGROUND INFORMATION AND REGULATORY INCENTIVE TO PURSUE DISSOLUTION TESTING.”
Over the last couple of decades there have been ongoing efforts to define appropriate methods for orally inhaled product (OIP) dissolution testing, but there are currently no compendial specifications. However, in recent years, the US FDA has released several Product-Specific Guidances (PSGs) that highlight dissolution testing as part of an enhanced suite of in vitro methods that can be deployed to eliminate the requirement for a clinical endpoint study – an important development. As a result, today’s OIP developers find themselves with a growing body of background information and regulatory incentive to pursue dissolution testing. A clear understanding of the challenges and opportunities is essential to navigating this evolving landscape.
MOTIVATIONS FOR OIP DISSOLUTION TESTING
While much attention is often given to the potential of pulmonary drug delivery as a route for systemic drug delivery, the reality is that OIPs for the localised treatment of respiratory illness dominate the commercial marketplace. At least a quarter of a billion people worldwide are estimated to be living with asthma alone, and it is still under-diagnosed and under-treated, notably in low- to middle-income countries.1 More effective and inexpensive OIPs for the treatment of respiratory illness are a high priority for healthcare providers the world over.
Against this backdrop, three motivations for dissolution testing can be identified:
- To accelerate the development of generic products
- To improve the efficiency and efficacy of OIPs for localised action
- To optimally employ the pulmonary route for systemic drug delivery.
Demonstrating Bioequivalence
The FDA’s strategy of releasing PSGs “to facilitate generic drug availability”2 is progressively establishing clearer pathways for the demonstration of bioequivalence (BE) for a growing number of OIPs. Most of these products are locally acting, a complicating factor in clinical endpoint studies that can lead to unreliable and/or unpredictable data. Given that such studies are also time-consuming, recent PSGs outlining a streamlined pathway almost exclusively reliant on in vitro methods are welcome.
“AS RELIANCE ON IN VITRO DATA INCREASES, SO TOO DOES THE NEED FOR RIGOUR WITH RESPECT TO CAPTURING ANY POTENTIAL FOR CLINICALLY SIGNIFICANT DIFFERENCE.”
This alternative pathway calls for seven in vitro studies to demonstrate BE, expanding on the traditional battery, by incorporating realistic aerodynamic particle size distribution (APSD) and dissolution testing. As reliance on in vitro data increases, so too does the need for rigour with respect to capturing any potential for clinically significant difference. Given the time and cost benefits of eliminating a clinical endpoint trial, these PSGs provide a strong incentive for the implementation of dissolution testing within OIP development.
Developing a Better Understanding of In Vivo Behaviour
Beyond its regulatory value, dissolution testing is becoming increasingly important as a tool to better understand what happens to inhaled drug particles post-deposition, and how to control this behaviour in ways that enhance local efficacy and/or systemic drug delivery.
The overarching goal for pulmonary drug delivery is to disperse the dose to a respirable particle size, with 5 µm typically stated as the upper limit for pulmonary deposition. This explains the critical role of APSD measurement. Digging deeper, it is known that the APSD of the <5 µm fraction influences regional deposition within the lung and that the targeted deposition needs to be matched with the mechanism of action of the drug to maximise therapeutic effect.
For example, studies with three monodisperse salbutamol aerosols (mass median aerodynamic diameter (MMAD) -1.5, 2.8 and 5 µm) have demonstrated that, for patients with mild to moderate asthma, an MMAD of 2.8 µm is optimal with respect to bronchodilation. This is consistent with the understanding that finer particles (<5 µm) can penetrate the small airways where receptors for salbutamol are concentrated.3
Knowledge of where to deposit different drugs optimally, and how to do so, although incomplete, far exceeds understanding of the next steps towards therapeutic action. There are three possible fates for particles deposited in the lung:
- Dissolution and absorption, ultimately into systemic circulation
- Degradation via drug metabolism
- Removal via mucociliary clearance.
The relative rates of these processes are critical considerations for those seeking to:
“A GROWING PUSH TO DELIVER HIGHER DRUG LOADS (ESPECIALLY FOR SYSTEMIC DRUG DELIVERY), INCREASING NUMBERS OF POORLY SOLUBLE DRUG CANDIDATES AND INTENSE ACTIVITY TO REALISE INHALED BIOLOGICS FURTHER AUGMENT THE VALUE
OF DISSOLUTION TEST DATA.”
- Extend the residence time of locally acting drugs at the site of action
- Accelerate and maximise systemic uptake, or, conversely, to minimise it
- Maximise the fraction of the delivered dose that has a clinical impact, relative to that lost/wasted.
Such goals align with efforts towards greater efficacy, improved cost-efficiency and effective systemic delivery. A growing push to deliver higher drug loads (especially for systemic drug delivery), increasing numbers of poorly soluble drug candidates and intense activity to realise inhaled biologics further augment the value of dissolution test data.
OSD DISSOLUTION: LESSONS FOR OIPS
Of course, the pharmaceutical industry is not new to dissolution testing. Therefore it is reasonable to see what can be learned from experiences of testing oral solid dosage (OSD) forms. The United States Pharmacopoeia (USP) Dissolution Methods Database4 highlights the key parameters defined for methods for dissolution testing for OSDs and other pharmaceutical product forms. These include:
- Apparatus design
- Dissolution medium (e.g. composition, quantity, temperature aeration state)
- Apparatus-specific parameters (e.g. flow rate, agitator speed)
- Timeframes for testing (i.e. sampling times and total test time)
- Assay method (for quantifying dissolved drug).
In combination these factors define:
- How the sample and dissolution medium contact with one another
- The extent to which the dissolution medium mimics the clinically relevant chemical characteristics of the in vivo environment
- Whether testing is carried out under sink conditions or not
- The hydrodynamic regime applied during testing
- The sensitivity with which the concentration of dissolved drug can be detected
- The ability to robustly track the dissolution process (i.e. fast or slow).
All these issues are equally pertinent to OIP dissolution testing and can provide a valuable starting point as methods continue to evolve.
REPRODUCIBILITY VERSUS CLINICAL REALISM
The overarching context in any in vitro method development is the balance between reproducibility and clinical realism. Generally, improving clinical realism increases the complexity of a technique, which can reduce reproducibility and, by extension, differentiating capabilities. Given that OIP performance is further impacted by a range of patient-specific factors, there is also the risk that improving clinical realism for one patient population reduces it for another.
Debates around whether to apply sink conditions during OIP testing illustrate this point. Typically, OSD dissolution testing is carried out using a volume of dissolution medium at least three times greater than that required to form a saturated solution of the drug substance. Under these “sink” conditions, the bulk concentration of drug in the dissolution medium is too low to affect dissolution rate, eliminating a potentially confounding factor. For most OSD drugs, sink conditions are clinically relevant.
By contrast, the pleural fluid film that covers the surface of the lung amounts to a total volume of just 10–20 mL in a healthy patient.5 The likelihood of sink conditions being clinically relevant is therefore questionable. While the volume of fluid into which the drug dissolves is small, the subsequent rate of drug absorption is also relevant. Theoretically, with sufficiently high permeability or absorption, sink conditions could prevail, despite the low fluid volume.
What can be asserted is that testing under sink conditions will deliver test data unaffected by bulk concentration, increasing the chances of good reproducibility. Testing with far lower volumes of dissolution medium may increase clinical relevance – or not. True clinical relevance would call for testing under conditions that accurately simulate the amount of fluid present, taking into account any change associated with disease state and, at the same time, allowing for permeability differences of the drug. The result: a more complex test set-up, greater scope for variability and, ultimately, lower reproducibility.
In a similar vein, there may be difficult choices to make over the composition of the dissolution medium. Options range from simple phosphate buffered saline to simulated lung fluid, which is usually a solution of minerals and salts with or without added surfactant. In vivo, the composition of lung fluid varies across lung regions and may also be impacted by disease state,6,7 adding further complexity to the method design.
SURVEYING THE OPTIONS FOR OIP DISSOLUTION TESTING
A first point to note when considering options for OIP dissolution is that there are few commercially available solutions for those looking to establish in-house testing. Many of the experimental set-ups reported in the literature are custom made for specific studies. Nevertheless, the majority can be classified as follows (Figure 1):
- Paddle Over Disk: Modified versions of USP Apparatus 5
- Flow Through Cell: Modified versions of USP Apparatus 4
- Diffusion-Controlled Cell: Vertical Diffusion Cell or Transwell.
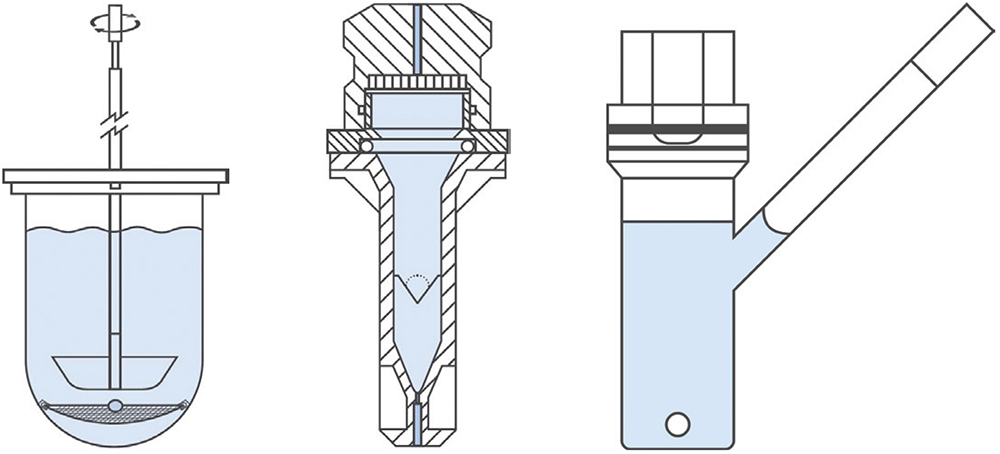
Figure 1: Schematics illustrating the three different types of apparatuses routinely used for OIP dissolution studies. From l to r: paddle over
disk, flow through cell, and vertical diffusion cell.
“FPD IS A WELL-ESTABLISHED METRIC FOR OIP CHARACTERISATION, SO THIS APPROACH IS A GOOD STARTING POINT WITH RESPECT TO BOTH CLINICAL RELEVANCE AND ALIGNMENT WITH EXISTING PRACTICES.”
Regardless of apparatus choice, dose collection is an important first step. Here, consensus has converged on use of the fine particle dose (FPD), typically the sub-5 µm dose as determined by cascade impaction.6–8 FPD is a well-established metric for OIP characterisation, so this approach is a good starting point with respect to both clinical relevance and alignment with existing practices.
Use of the FPD requires careful consideration of sample transfer from the dose collection device to the dissolution testing apparatus. Various solutions have been developed, but it is important to:
- Recognise the potential to disturb the precise aerodynamic regime in an impaction device
- Ensure effective transfer and consistent positioning within the dissolution apparatus.
Returning to dissolution testing apparatus choice, diffusion-controlled cells hold the sample in a far smaller volume of fluid than alternative apparatuses, a favourable point with respect to replicating conditions within the lung. However, results can be difficult to interpret, notably the relative rate of diffusion and dissolution, and highly dependent on the properties of the selected membrane, as diffusion is the controlling mechanism.6,7
Paddle over disk and flow through cells enable the application of sink conditions. With paddle over disk, it is also possible to reduce the dissolution medium volume deliberately to simulate the impact of drug concentration build-up. Possible issues associated with the use of paddle over disk apparatuses include the potential for dead space beneath the sample holder and inconsistencies in sample positioning within the dissolution vessel. The need to ensure wetting of the sample can be met through appropriate membrane selection and choice of dissolution medium. Wetting can likewise be an issue with flow through cell designs, which can also suffer from air build-up around the sample holder, flow gradients and other flow rate-related dissolution effects.6,7
MEETING CURRENT OIP TESTING REQUIREMENTS
A key question in OIP dissolution testing is the extent to which any proposed method satisfies the requirements set out in PSGs, to “demonstrate discriminatory ability (e.g. ability to detect meaningful differences in formulation or manufacturing process, such as a difference in deposited drug particle size) in measuring the dissolution kinetics of the product”.9
Within this context, there is good reference data comparing the dissolution behaviour of fluticasone propionate (FP) delivered by metered dose inhaler (MDI) and by dry powder inhaler (DPI). Here, we have datasets made with different apparatus types and test methods.10–12 All identify DPI-delivered particles as dissolving faster than those delivered by MDI, despite published APSD data indicating that MDI analogues deliver finer particles.12 There is also evidence that this difference in dissolution rate correlates with observed differences in in vivo absorption rate,10 highlighting the value of dissolution testing for OIPs.
Figure 2 shows data exemplifying the device-dependent dissolution behaviour of FP and illustrating a further factor in OIP dissolution testing – the effect of drug product loading. The high surface area of the lung, relative to that used for dose collection, make loading effects relatively unlikely in vivo. Conversely, the tendency for a sample to distribute non-uniformly on the collection surface of impaction devices, notably beneath nozzles, compounds the likelihood of drug-loading issues during FPD collection.
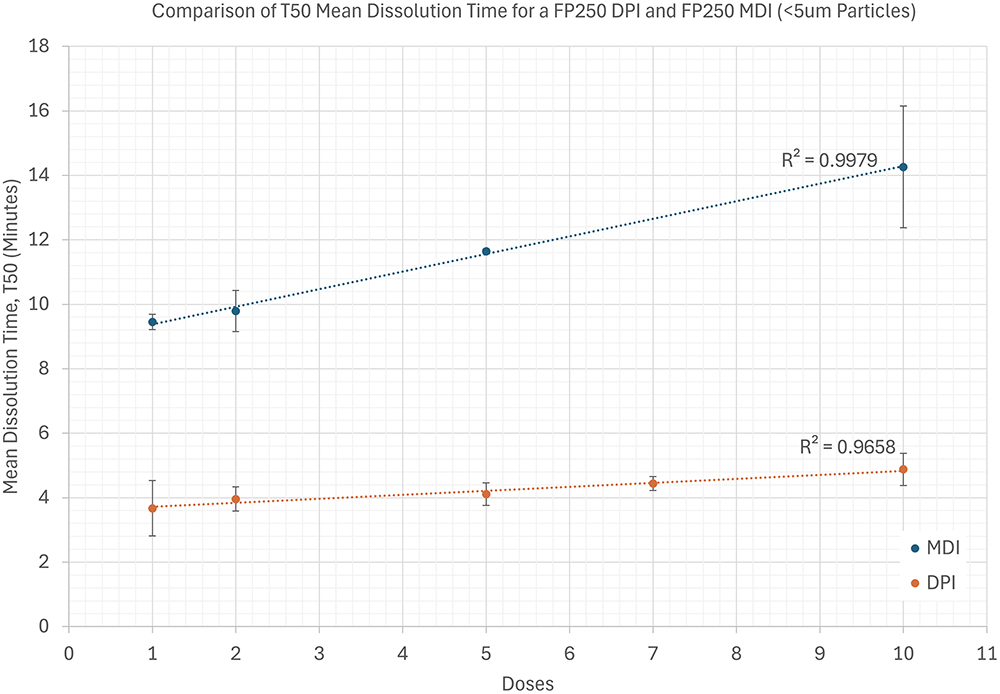
Figure 2: Example data contrasting the dissolution behaviour of FP delivered by MDI (Evohaler, GSK) and DPI (Accuhaler, GSK) measured using a paddle over disk apparatus for OIP dissolution testing (Inhaled Dissolution Apparatus, Copley Scientific).
In the dataset shown, there is some dependency on dose number, notably with the MDI, which has a higher FP recovery per dose; ten actuations of the MDI equate to a deposited dose of 800 µg of drug; the equivalent figure for the DPI is 500 µg. However, such effects are far outweighed by the degree of differentiation between the devices. Minimising the number of actuations within the constraint of assay viability – a standard approach for other forms of OIP testing – is a sensible strategy for mitigating such effects and maximising the method’s capability for robust differentiation.
LOOKING AHEAD
Given the growing number of PSGs, OIP dissolution testing is likely to become more common, and there are already signs of its application for nasal drug products too. Developers looking for appropriately differentiating methods have commercially available equipment to use and valuable data to reference, as discussed here.
Beyond that, pioneers in OIP dissolution continue to advance test set-ups incorporating, for example, mucous gel layers and/or cell-based monocultures to better bridge in vitro to in vivo.8 While not yet practical for routine testing, such solutions have the potential to elucidate our understanding of the in vivo processes to optimally deploy pulmonary drug delivery for the widest possible array of applications. Lessons already learned with OIPs will help to accelerate uptake in the nasal drug product arena, where opportunities for systemic delivery demand equally rigorous and insightful approaches.
REFERENCES
- “Asthma”. World Health Organization, May 6, 2024.
- “Product-Specific Guidances for Generic Drug Development”. US FDA, Mar 2025,
- Labris NR, Dolovich MB, “Pulmonary drug delivery. Part 1: Physiological factors affecting therapeutic effectiveness of aerosolized medications”. Br J Clin Pharmacol, 2003, Vol 56(6), pp 588–599.
- “Dissolution Methods Database”. USP, accessed Oct 2025.
- D’Agostino HP, Edens MA, “Physiology, Pleural Fluid”. StatPearls Publishing, Jan 2025.
- Riley T et al, “Challenges with Developing In Vitro Dissolution Tests for Orally Inhaled Products”. AAPS PharmSciTech, 2012, Vol 13(3), pp 978–989.
- Nokhodchi A, Chavan S, Ghafourian T, “In Vitro Dissolution and Permeability Testing of Inhalation Products: Challenges and Advances”. Pharmaceutics, 2023, Vol 15(3), p 983.
- Son Y-J et al, “Optimization of an In Vitro Dissolution Test Methods for Inhalation Formulations”. Dissolution Technologies, May 2010.
- “Draft Guidance on Fluticasone Propionate”. US FDA, Aug 2024.
- Price R et al, “Development of an Aerosol Dose Collection Apparatus for In Vitro Dissolution Measurements of Orally Inhaled Products”, AAPS J, 2020, Vol 22(2), p47.
- Huang A et al, “An In Vitro Dissolution Method for Inhaled Drugs Depositing in the Tracheobronchial Lung Region”. Pharm Res, 2025, Vol 42(4), pp 639–650.
- Huang A, “A Tracheobronchial Specific in vitro Dissolution Test – A Problem Well Filtered is a Problem Half Dissolved”. MSc Thesis, University of Alberta, 2025.

