To Issue 182
Citation: Khanolkar A, Neumann A, “Early Automation Considerations for the Development of Drug Delivery Combination Products”. ONdrugDelivery, Issue 182 (Jan 2026), pp 94–98.
Visit SMC at Pharmapack Paris! – Stand 4G63
Asmita Khanolkar and Al Neumann uncover the manufacturing strategies that can be used to achieve design for automation, scale-up and sustainable solutions for clinical and commercial launch, describing the gains to be made in manufacturing efficiency when using small, automated work cells.
Today’s novel drug delivery devices are complex, tailored, targeted and customised to meet the requirements of challenging applications. A robust manufacturing plan is needed from the first design input in order to successfully achieve automation for a scale-up strategy. In turn, this accomplishes integration of development through clinical and commercial manufacturing.
“THE FOCUS TODAY IS ON PROVIDING PATIENT-CENTRIC TECHNOLOGY SOLUTIONS FOR PERSONALISED AND PRECISION MEDICINE, WHERE ONE SIZE DOES NOT FIT ALL.”
The CMC (Chemistry, Manufacturing, and Controls) side of the programme can have a huge impact on the timeline and costs of development. Iterations for both the drug and device are inevitable and ever faster turnaround is needed throughout development, manufacturing and testing cycles. Automated manufacturing plays a central role here.
Drug delivery devices cover a wide range of innovative solutions from autoinjectors and on-body devices to complex reconstitution devices, covering needs across parenteral injection, respiratory, transdermal and ophthalmic delivery solutions (Figure 1). The focus today is on providing patient-centric technology solutions for personalised and precision medicine, where one size does not fit all.
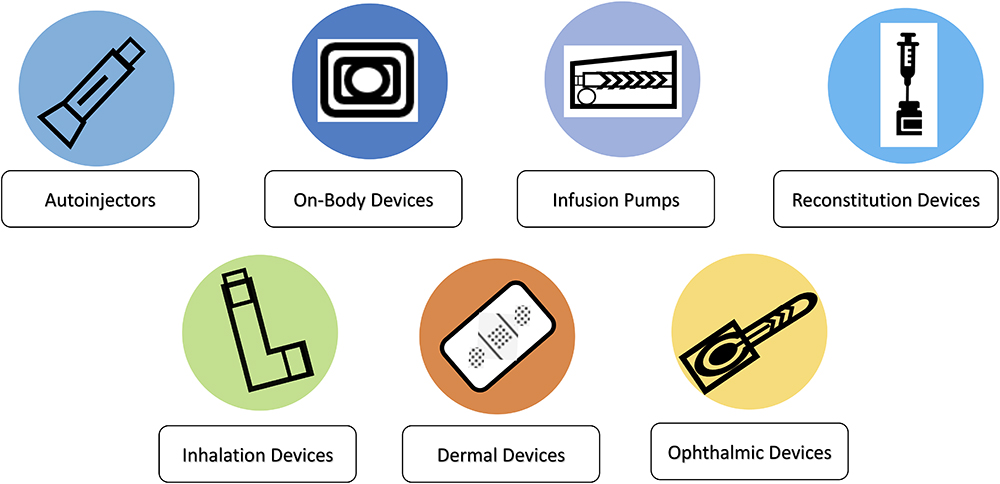
Figure 1: Innovative solutions make up today’s complex drug-delivery devices.
For combination products, manufacturing strategies need to cover the device build as well as drug handling aspects, taking the CMC requirements into account. This can often be challenging as it requires broad expertise in various aspects of moulding, assembly, automation, fill-finish and packaging.
Especially when focusing on developing a novel therapy or drug chemistry, the delivery device and its manufacturing can become an afterthought. Automation and scalability can end up being considered further down the line too. The starting point of device manufacture is understanding all the steps from early development to commercialisation.
“DEVICE MANUFACTURING INVOLVING COMPLEX ASSEMBLY PROCESS STEPS AND CUSTOM REQUIREMENTS CAN ALSO BENEFIT FROM DESIGN FOR AUTOMATION FROM AN EARLY STAGE.”
This can be missed, particularly when considering the interactions and touch points of the drug and device necessary to achieve overall project success. Maintaining consistent product performance throughout manufacturing scale-up in a product’s lifecycle is crucial. Designing for automation can help developers to focus on this from the very beginning, by examining complex assembly steps and customer requirements. With the stringent requirements of speed to clinic, a timeline for development can be greatly streamlined by putting early efforts into design for automation, as well as a manufacturing strategy. Moreover, device manufacturing involving complex assembly process steps and custom requirements can also benefit from design for automation from an early stage.
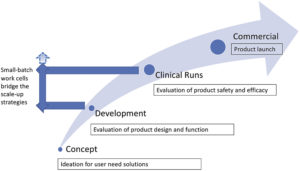
Figure 2: The need for small-batch, semi-automated work cells.
MANUFACTURING STRATEGY
A manufacturing strategy starts with the scale requirements for the product. Depending on the application, the commercial product launch volumes can range from low to high. Low volume quantities are needed for development and clinical studies, and there is typically a steep jump to high commercial quantities. Suggestions that can be made early in the project include developing small work cells for repeatability of assembly process steps. Further proposals can cover product design and test recommendations to help with future, higher throughput equipment.
Small-batch work cells remain more flexible as product development evolves, while also supplying enough product for testing and market analysis at lower work cell costs. Additionally, they provide an opportunity to develop testing strategies and to explore techniques for assembly, work cell component handling and programming routines that can be adapted to higher throughput machines. In most cases, small work cells can help to expose any new product deficiencies and allow for early part modifications (Figure 2).
Design for Automation
Design for automation principles cover various areas of assembly, including standardisation, minimising component use, handling features, assembly orientations, self-alignment, tolerance stack-up and process optimisation. When it comes to assembly of complex devices, the focus is on de-risking the process and removing uncertainties, such that the product can be repeatedly manufactured in the same way for consistent device performance.
For drug delivery devices, successful clinical outcomes – in terms of pharmacokinetics and pharmacodynamics – depend on optimised and consistent delivery,1 therefore constant high performance becomes the driving factor for success. Device design outputs for delivery performance are included in the US FDA’s recommendations for establishing and assessing drug delivery performance via essential drug delivery outputs (EDDOs).2 By incorporating design for automation, design outputs can cover the manufacturing and testing specifications in addition to the device design specifications, in order to meet the EDDO recommendations (Figure 3).
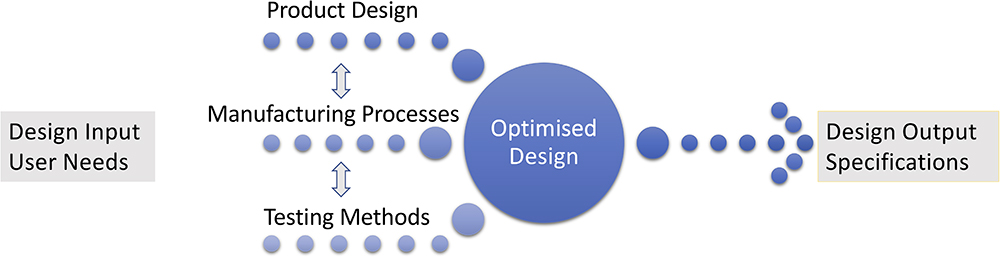
Figure 3: Design, manufacturing and testing outputs for optimised design.
The design of small-batch, automated work cells focuses on optimised steps that can de-risk the process. There are many areas that can pose risks to the final product performance. A good first step is to look at the process, in order to evaluate steps that are critical and where early automation makes the most sense. These can include the handling of expensive components; challenging manual assembly steps that are not consistently carried out; custom assemblies, blind assemblies; multi-step assemblies; process steps that are difficult to test or must be quantified with certain parameters, time-intensive, time-sensitive or stringent requirements; and complicated joining, welding or gluing combinations.
Additionally, secondary operations, such as custom printing, packaging and labelling, can also pose manufacturing challenges and are better accomplished via automation in small work cells. Furthermore, when considering operator safety, steps that take an inordinate amount of dexterity can benefit from levels of automation.
Component Handling
Component work cells can be used to orientate, print and handle challenging components. In the case of sharps and needles, these components may need to be presented one at a time in certain orientations. With the aid of vision systems, dimensional or visual inspection can be conducted prior to assembly. Advanced laser marking technologies can add value by pre-marking certain componentry while avoiding any loss at a later, more expensive assembly stage.

Figure 4: Automation of assembly steps incorporating force, distance and torque monitoring.
Device Assembly
Various assembly steps comprise of push, press, snap-fit or turning operations. Even though these operations may seem simple for manual assembly, process controls can be essential for performance, and data may need to be collected during critical assembly steps. In such cases, monitoring forces, displacement and torque can generate in-process data to assure repeatability in the assembly steps (Figure 4).
“FOR OPERATIONS REQUIRING JOINING METHODS SUCH AS ULTRASONIC WELDING, LASER WELDING OR GLUING, CUSTOM SMALL- ATCH WORK CELLS CAN HELP TO REMOVE UNCERTAINTIES IN THE PROCESS.”
For operations requiring joining methods such as ultrasonic welding, laser welding or gluing, custom small-batch work cells can help to remove uncertainties in the process. Furthermore, part detection can identify any missing components. Glue dispensing and UV-curing is a common assembly operation; the quantity of glue and cure timing are important parameters to monitor. In this process, needle orientation, straightness and height post-gluing become critical parameters. Process data available from the work cells can help optimise the gluing process for consistent performance.
Ultrasonic welding, laser welding and other joining assembly methods rely on in-process controls since the final testing is often destructive. Due to this, it becomes imperative to collect data throughout the process. Some of the parameters that can be monitored include the cycle time, cycle completion verification and pass/fail statistics via machine vision testing. Operator ergonomics and safety regulations for a manufacturing environment can be incorporated into such small work cells (Figure 5).

Figure 5: Operator-friendly semi-automated stations for assembly.
Testing
Testing can be an integral part of a small work cell or a stand-alone station (Figure 6). It is important to identify the controls needed for each process and to set up relevant testing methodologies early on.
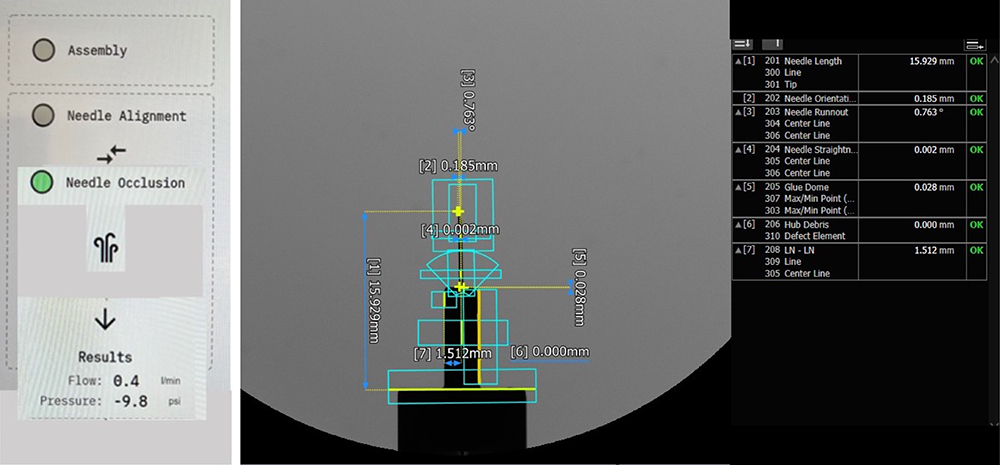
Figure 6: Testing concepts for needle alignment and occlusion.

Figure 7: Testing station with reject sorting.
Custom leak, flow and pressure decay tests can help identify important failure modes, such as occlusion (Figure 7). Such methods can be validated to leak standards and can provide important insights into flow path occlusion as parts are assembled. Data collected during testing can help troubleshoot any issues with tolerance stack-ups or identify manufacturing defects early on. Machine vision provides additional insights into product quality and conformity. Figure 8 shows a summary of possible capabilities that can be automated.
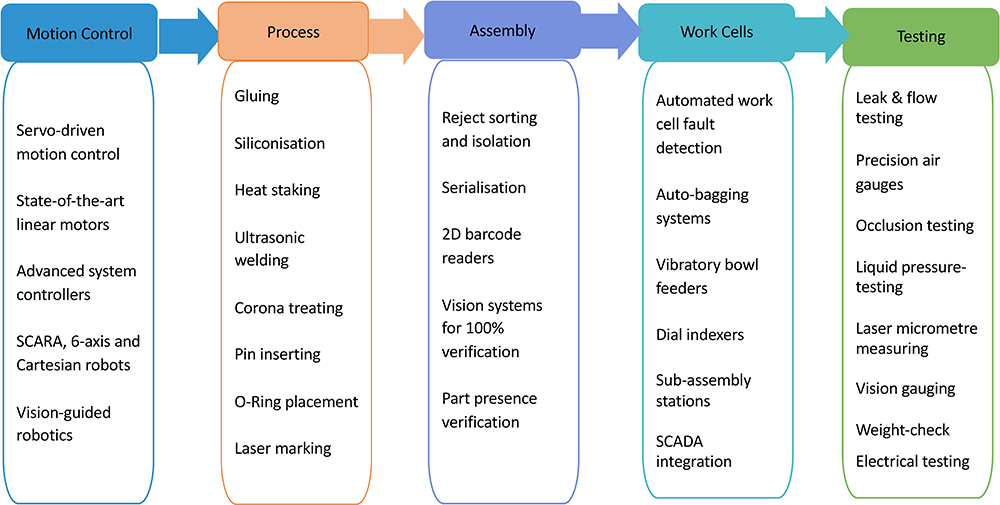
Figure 8: Some of the capabilities’ matrix available for automation into work cells.
Packaging & Labelling
Packaging and labelling work cells can significantly help to align the device development steps towards a finished product. Protecting a product and maintaining its sterility are key packaging requirements, and automation of the sealing, printing and labelling processes can help minimise the loss of expensive product.
For drug products that require cold storage, the process steps for device assembly and secondary packaging must incorporate careful handling of temperature-sensitive drugs or drugs with short shelf life. Real-time monitoring of process parameters in work cells can help de-risk handling of sensitive drug products. Semi-automated packaging work cells can also help to incorporate sustainable packaging solutions and handling of delicate packaging materials.
Pilot Line
The design for automation concepts in small work cells can be put together to run pilot-scale volumes (Figure 9). Depending on the quantity requirements, the line can be set up as modular stations or implemented in a rotary or linear line. Building guidelines for automation should be defined based on industry-approved standards.
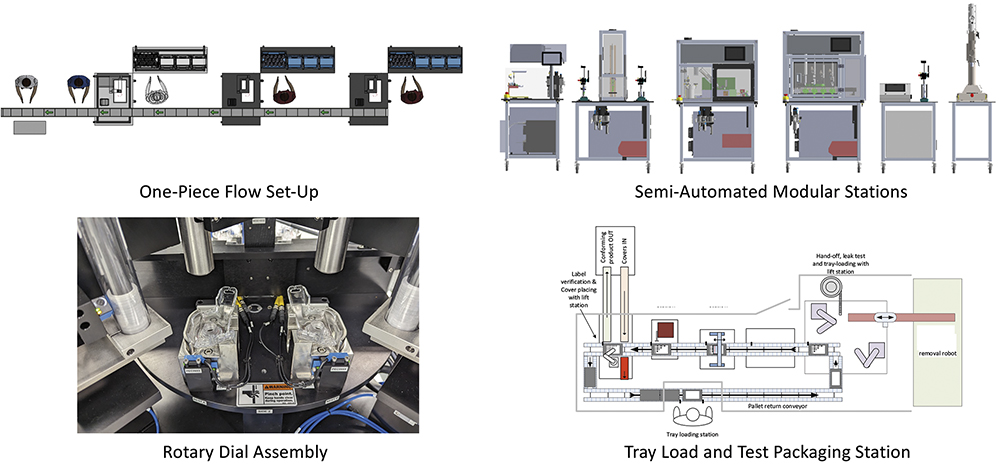
Figure 9: From manual to semi-automated stations for a pilot line (up to 5 ppm).
HIGH-SPEED/HIGH-VOLUME LINES
The findings from small-batch work cells in pilot line concepts are used to map high-volume, high-speed automation lines. Without the working knowledge of small-scale automation steps and process parameters, large-scale production lines can become very expensive and risky to build. As artificial intelligence and machine learning tools become more sophisticated, data collected from process parameters through small stations and pilot lines can provide relevant information and insights for de-risking a high-volume line.
CONCLUSION
In summary, automation in small work cells can help to increase speed to clinic and de-risk development. The data collected through small work cells can ensure consistent performance and help to avoid surprises during clinical testing due to manufacturing inconsistencies. Input from manufacturing, quality, regulatory and clinical teams involved with early manufacturing strategies can help to align a well-balanced CMC strategy for development of complex drug delivery devices and combination products. Designing for automation early on not only improves the manufacturing consistency of clinical supplies but also brings additional value to future high-speed and high-volume assembly systems.
REFERENCES
- Khanolkar A, White S, Margerison E, “A Novel Method to Optimize Drug Delivery for Parenteral Products Involving New Therapies and Unmet Needs”. Pharm Res, 2023, Vol 40(10), pp 2303–2315.
- “Draft Guidance for Industry: Essential Drug Delivery Outputs for Devices Intended to Deliver Drugs and Biological Products”. US FDA, Jun 2024.

