To Issue 138
Citation: Chandra A, Gross C, “A High-Quality and Robust Safety System for High-Volume Biologics at Home”. ONdrugDelivery, Issue 138 (Oct 2022), pp 38–41.
Audrey Chandra and Cécile Gross discuss Nemera’s development of a new product for the safe delivery of high-volume biologics at home.
“Home-care administration brings many benefits, lowering the cost of treatment for all stakeholders and increasing adherence to regimens.”
Needles have been used to inject drug treatments for a long time, via intradermal, subcutaneous or intramuscular routes. Thanks to prefilled syringes (PFSs), a number of new drugs are now being administered in medical settings. These include different types of molecules – for example, anti-thrombotic agents, such as heparin. Vaccines and other small molecules are also commonly administered subcutaneously (SC) by healthcare professionals. However, recent developments in drug research and production have made available bigger molecules. Biological drugs have become available in PFSs and are predominantly used to treat complex chronic conditions.
The trend towards self-administration comes from two directions at the same time: from pharmaceutical companies that offer these biological drugs for chronic conditions in PFSs – and patients themselves.
Intravenous (IV) treatments involve patients going to healthcare facilities to receive their injection, as handling PFSs or IV administration would be too tricky and dangerous for novice users at home. The risk of needlestick injuries and the wrong use of the device are considered too high, leading to compromised patient safety. Both cases therefore require patients to travel to clinics or hospitals on a regular basis, leading to frequent hospital chair time for patients, also contributing to the burden on hospitals. Therefore, pharmaceutical companies are striving to move injectable drugs from IV to SC, hence avoiding hospital chair time for patients or long and inconvenient hospitalisation.
In parallel, and following the covid-19 pandemic, patients have ceased to visit their healthcare professionals and got used to teleconsultations. Some patients even became reluctant to go for their regular medical visit at the hospital. They preferred to manage their drug regimen with reassurance at home, without having to worry about being exposed to the virus.
Home-care administration brings many benefits, lowering the cost of treatment for all stakeholders and increasing adherence to regimens. Self-administration in a home-care setting becomes more and more common, leading to increased risk of use errors and sharp injuries to be expanding concerns for patients’ safety. With this in mind, protecting users from sharps injuries whilst optimising the injection experience has become a must. Patients or caregivers need reliable, robust and easy-to-use devices to administer their treatment on a regular basis at home, independently and with optimised convenience.
SAFETY SYSTEM FOR BIOLOGICAL SELF-ADMINISTRATION
How did Nemera enter this market? It first offered a safety system with a Luer lock, but the availability of PFSs with a staked needle has made it possible to offer the first Safe’n’Sound® in 1 mL. Compatible with several kinds of glass PFS following ISO standards, it is also compatible with the PLAJEX® polymer PFS from Terumo.
In order to add the benefits of a platform approach, the device has been conceived as flexible. Available in different kinds of flange types, it can offer customisation with “soft-touch” material to improve grip, as well as an overcap to ease the rigid needle shield removal. Colour customisation for a safer drug or dose identification is also available.
Features also include a large thumb pad for ease of use, clear visibility for easy drug inspection and a rounded shape for an increased labelling surface. As for product performance, robustness has been the focus of the entire design. As a result, the full body in polycarbonate and the plunger rod in polypropylene with a large thumb pad and large finger flanges enable one-handed injection, no bending of any parts and good resistance in drop tests.
Safe’n’Sound® has been fully validated through user studies and is already commercially available with market references in Europe and the US. To facilitate its partners’ choice of assembly tools, Nemera has also bound partnerships with industrial providers that have gained full knowledge of the product characteristics and can be recommended.

Figure 1: The Nemera Safe’n’Sound® 2.25 mL safety system.
SAFE’N’SOUND® 2.25 ML: A CUSTOMISABLE PLATFORM
Biological drugs are very often needed in high concentration. This can be driven by the nature of the molecule, the composition of the final drug and the effort to decrease the frequency of treatment for the patient. Their viscosity increases according to the power law of the antibody concentration. Thus, to be injected, these molecules need to be diluted, which leads to higher injection volumes and lower (but still high) viscosity. As a result, larger-dose drug deliveries are a growing segment, with volume shifting towards 2 mL.
Taking this market need into account and leveraging its experience with the 1 mL version, Nemera developed a 2.25 mL device, safeguarding the device robustness as well as the platform approach (Figure 1).
Increasing the volume to inject has not compromised the ergonomic design, which makes the Safe’n’Sound® 2.25 mL intended for naïve users, including patients with dexterity issues, as well as operable by experienced users and healthcare professionals alike.
“Differentiation for pharma partners and drug identification for patients are crucial elements that can be handled through colour coding.”
FOCUS ON PHYSICAL IMPAIRMENT
In immunology, for example, where biologics and biosimilars are often used to treat chronic diseases, patients often have dexterity issues due to symptoms triggered by the pathology itself, age or comorbidities. These chronic conditions include, but are not limited to, rheumatoid arthritis, psoriatic arthritis and multiple sclerosis. To help such patients manage the removal of the needle cap, Nemera has developed an overcap. This rigid needle shield remover allows an intuitive gesture for quick and safe removal, thanks to its ergonomic design. Users’ inputs have been integrated in the development process as early as possible to identify the key elements to take into account.
Differentiation for pharma partners and drug identification for patients are crucial elements that can be handled through colour coding. Thanks to its broad injection capabilities, Nemera is able to offer this customisation option as well as soft-touch material customisation with biomaterial injection.
FULLY AUTOMATED MANUFACTURING CAPABILITY
Nemera chose to invest in a new industrial line to address market needs. The choice to increase its footprint in Europe was guided by its desire to better serve European customers from a sustainability and transportation standpoint. From an industrial point of view, the plant benefits from the company’s long experience in managing parenteral projects and not starting from scratch. As for product quality, the goal was to implement a line capable of producing a “glass-like” quality product, requested by several regulatory pathways and by high-value biologics. Leveraging from the current line, the direction has been optimisation, especially in process and quality control.
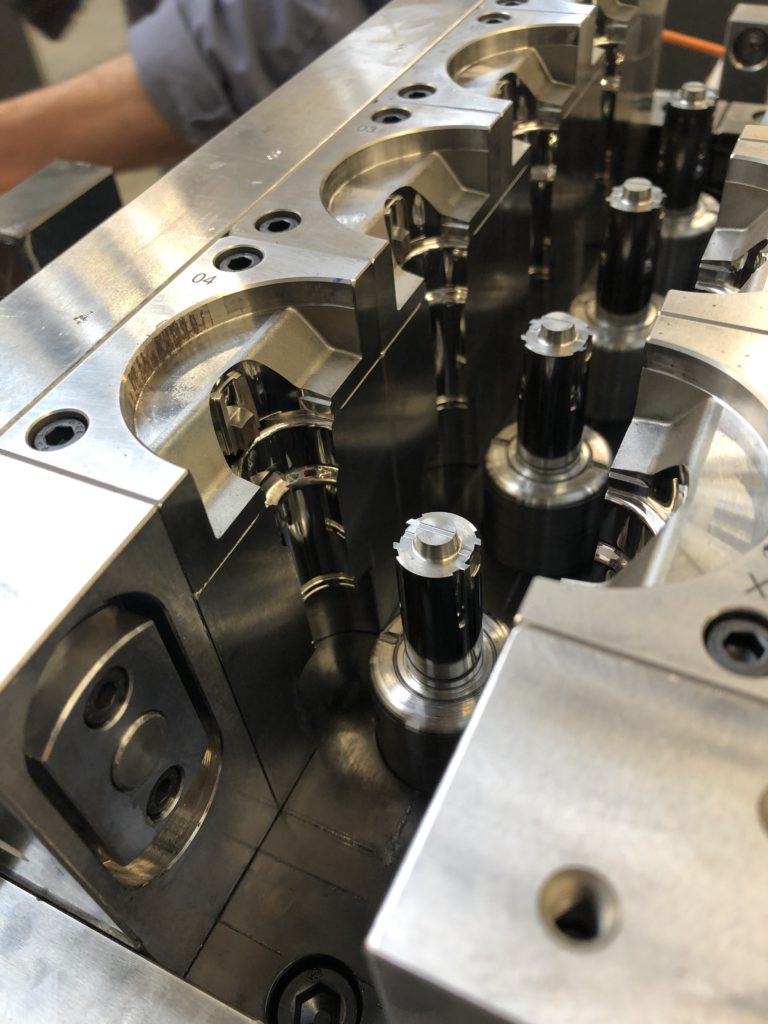
Figure 2: High-quality industrial line mould for glass-like safety devices.
As a result, this new line is equipped with new sets of moulds and high-end machines (Figures 1, 2 and 3), which will be industrially ready by 2024. Nemera’s efforts for continuous improvement as well as capacity expansion are embodied in this new state-of-the-art industrial line to produce high-quality devices with automated processes, tight visual control, especially on critical areas, as well as labelling and automated storage. These will limit human handling and therefore particle contamination, eliminate scuffs and scratches and focus on assembly to ensure final locking and safety mechanism activation.

Figure 3: State-of-the-art industrial line assembly.
The 100% visual inspection and dimensional control will be performed twice on all outputs: after the injection process of the sub-components as well as before the assembly of the sub-components to constitute a complete device. During the whole process, the sub-components will be packed in a tote to facilitate transfer between the injection machines to the assembly ones, and the assembled devices in a thermoformed tray, bulk being out of the question.

Figure 4: Automated industrial line packing machine.
A FULL RANGE OF SERVICES
Through capabilities in design research, human factors engineering, user experience design, engineering, laboratory services and regulatory support, Nemera is uniquely positioned to offer all the support that customers require, leveraging its Insight Innovation Center’s consulting expertise. Its service programme includes:
- Laboratory and analytical services to support characterisation and compatibility of drug products, syringe candidates and devices such as Safe’n’Sound® to optimise their integration.
- Help defining user groups/populations and early-use-related risk-analysis activities to define the human factors and usability programme necessary for a client’s regulatory/filing strategy and identified clinical risks.
- Developing instructions for use, value-added packaging, connectivity add-ons to support patient engagement/adherence and integration of training devices into the patient services model.
- Conducting formative and summative usability testing globally for all aspects of the device and supporting assets in alignment with the human factors programme definition through human factors engineering report documentation for use in regulatory submissions.
- Programme management excellence to ensure all elements of the programme are integrated to drive efficiency and mitigate any programme risks proactively.
“Ensuring patient safety and fostering patients’ adherence to therapies are at the heart of everything Nemera does.”
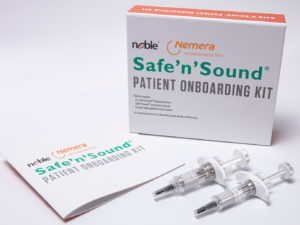
Figure 5: Nemera-Noble collaboration.
TRAINING TOOLS
Nemera collaborates with Noble to incorporate Safe’n’Sound® patient onboarding kits to allow patients to repeatedly practise the self-administration process (Figure 5). This partnership allows Noble to support Nemera’s launch of the new premium add-on passive safety device, by incorporating a robust training platform programme in tandem with the drug delivery device. The training solution is designed to replicate the Safe’n’Sound® 1 and 2.25 mL in form and function, which will allow for better assistance educating patients on the proper use of the device.
This capability combined with Nemera’s device platforms and manufacturing excellence helps customers achieve the outcome of a successful regulatory submission and commercial launch of safe, effective and differentiated combination products while mitigating the risks associated with multiple partners who may not have expertise and experience in all the aspects critical for success.
Ensuring patient safety and fostering patients’ adherence to therapies are at the heart of everything Nemera does. Its ultimate goal is to bring superior value to patients and customers.

