To Issue 130
Citation: Kangastupa T, “A New Platform for Suprachoroidal Drug Delivery Using Standard Commercially Available Needles”. ONdrugDelivery, Issue 130 (Mar 2022), pp 37–41.
Timo Kangastupa discusses the potential of the suprachoroidal space for highly effective, specifically targeted drug delivery to the choroid and retina, and how Visionisti’s technology simplifies and standardises the procedure, enabling the use of standard commercially available needles for suprachoroidal delivery.
The ophthalmic sector is a rapidly growing part of the drug delivery industry. This is due to a number of factors, including ageing populations, greater awareness of ophthalmic conditions and increased pharmaceutical investment into ophthalmic research and development. With this growth comes new impetus to innovate on how we deliver drugs to the eye and to improve on our ability to target desired segments and increase the bioavailability of therapeutics.
“There are numerous potential applications for suprachoroidal delivery, including target indications such as neovascular age-related macular degeneration, diabetic retinopathy, diabetic macular oedema, uveitis and choroidal melanoma.”
SUPRACHOROIDAL DRUG DELIVERY
One exciting area of innovation has been delivery to the suprachoroidal space, a potential space between the choroid (the vascular layer of the eye) and the sclera (the white layer of the eye). The suprachoroidal space is an appealing route of delivery as it allows for a drug injected at the front of the eye to traverse the eye’s circumference and be delivered directly to the choroid and retina, which would otherwise require a more invasive intravitreal injection, implant or even surgery. This has the dual advantage of increasing efficacy, with delivered drug concentrations at the choroid and retina being potentially 10 times greater than those achieved by intravitreal injection,1 and enhancing safety, as the delivered therapy is largely compartmentalised away from non-target tissues.2
There are numerous potential applications for suprachoroidal delivery, including target indications such as neovascular age-related macular degeneration, diabetic retinopathy, diabetic macular oedema, uveitis and choroidal melanoma.3 Suprachoroidal delivery has also been put forward as an ideal candidate for the administration of novel gene therapies targeting retinal cells and the retinal pigment epithelium, which could otherwise require a surgical subretinal injection.1
However, successfully delivering therapeutics to the suprachoroidal space is no mean feat. Initial approaches to access the suprachoroidal space were surgical in nature. More recently, research has been conducted on delivery into the space via injection. Using a standard hypodermic needle is exceedingly challenging, as under most circumstances the suprachoroidal space is collapsed (due to intraocular pressure), meaning that the injecting physician must rely on tactile cues to know when the sclera has been penetrated. In response to this issue, it has been suggested that use of a microneedle of a length precisely matched to the sclera and conjunctiva would be the ideal solution to avoid an accidental intravitreal injection taking place instead of a suprachoroidal one.4
Whilst presenting clear advantages compared with other methods of drug delivery to the retina, injection into the suprachoroidal space comes with its own set of challenges. For example, performing suprachoroidal injections using microneedles requires additional training and timecompared with traditional intravitreal injection methods.5 To tackle these challenges and unlock the potential of suprachoroidal injection, Visionisti has developed a novel drug delivery platform that enables the use of conventional, commercially available hypodermic needles and standardisation of the procedure to maximise convenience and minimise difficulty for all parties.
VISIONISTI’S PLATFORM
Visionisti’s delivery platform consists of two parts: an adjustable adapter (Figure 1) and a needle guide (Figure 2). The adapter is a simple add-on device that transforms a conventional hypodermic needle into a suprachoroidal injection device by precisely controlling the exposed length of needle, while the needle guide stabilises the eye, keeps the eyelids open and standardises the injection procedure, ensuring that injections are always carried out with a predefined angle and distance from the limbus.
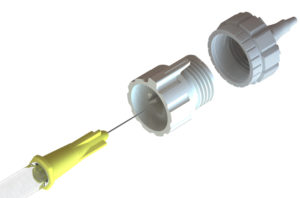
Figure 1: Visionisti’s adjustable needle adapter.

Figure 2: Visionisti’s needle guide.
Adjustable Adapter
Visionisti’s adjustable adapter is designed to fit on the end of commercially available hypodermic needles and provide easy and fast adjustment of their effective length. This is done by simply twisting the adapter, and it can be configured to allow for continuous, stepped or predefined levels of adjustment. In this way, Visionisti’s platform prevents accidental injection past the choroid and into the vitreous humour in much the same way that a specialised microneedle would, but with the advantage of using commercially available injection devices with standard hypodermic needles (Figure 3).
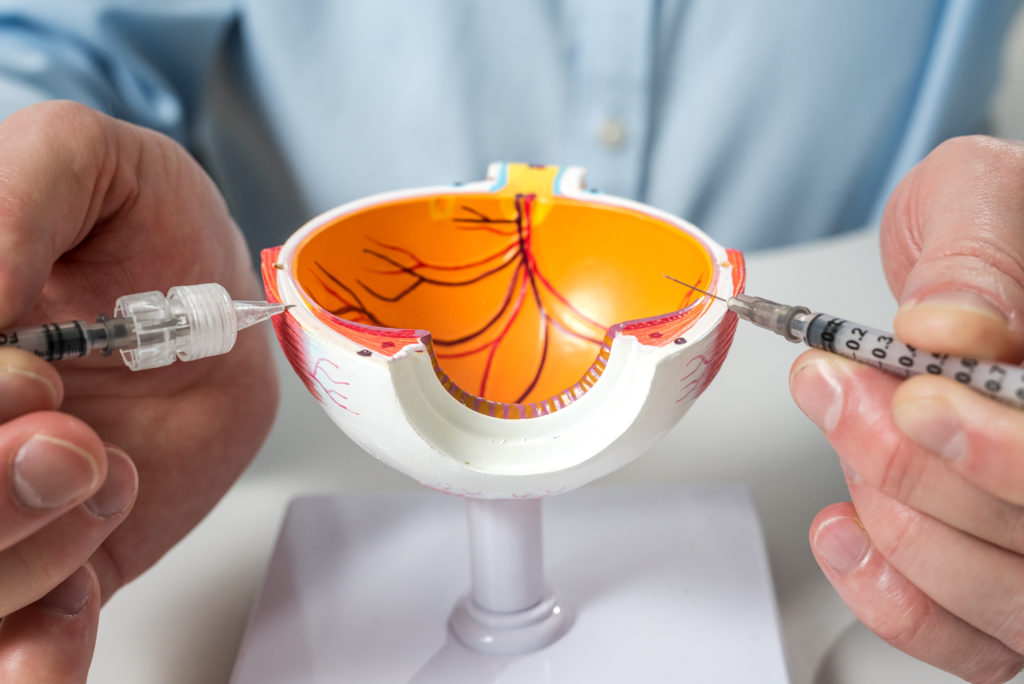
Figure 3: The adjustable adapter can make virtually any commercially available injection device suitable for suprachoroidal delivery by precisely controlling the length of the exposed needle, preventing accidental intravitreal injection (image credit: Elias Jokiranta Photography).
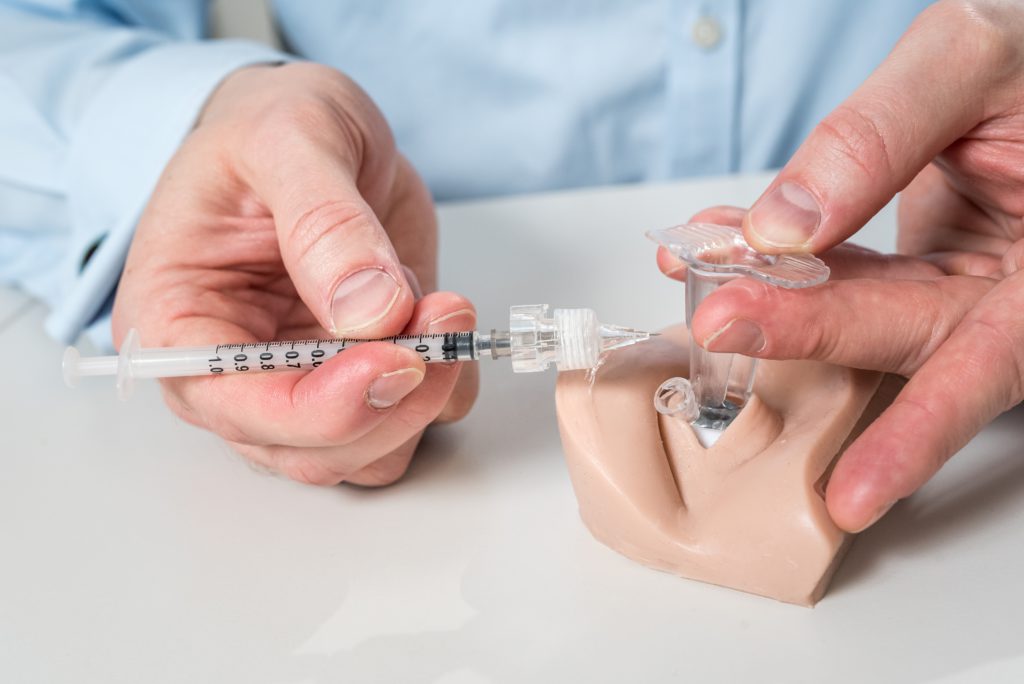
Figure 4: The needle guide maximises the convenience of the injection and standardises the procedure (image credit: Elias Jokiranta Photography).
The adapter is customisable and can be tailored to suit the needs of a specific ophthalmic injection device, including prefilled syringes, drug-coated solid needles and devices for injecting ophthalmic implants. As the adapter is an add-on to the injection device, it doesn’t come into contact with any drug product and has a minimal regulatory burden associated with it.
“The platform standardises the injection procedure, making it repeatable, faster and easier for the administering clinician.”
Needle Guide
The needle guide part of Visionisti’s platform is designed to standardise the suprachoroidal injection procedure. The guide is a cheap and disposable all-in-one tool to keep the eyelids open, stabilise the eye, control the injection angle and reduce injection pain. It also helps the needle track to close automatically, minimising the risk of endophthalmitis. Furthermore, the needle guide functions as a pressure plate after the injection, minimising the potential backflow. The guide is convenient to use, consisting of a single piece that is easy to put in place and keeps the eye focused forward, while an opening on the side controls the angle and approach of the needle (Figure 4).
An additional potential benefit being investigated is the possibility that some patients may experience reduced pain when the injection is administered with the needle guide. When the needle guide is applied to the eye, the pressure it exerts may have a paralysing effect on the ciliary nerves, resulting in a sensation of local anaesthesia.
Combined Platform
Together, Visionisti’s adjustable adapter and needle guide provide a complete platform for transforming commercially available needles into convenient, ready-to-use suprachoroidal injection devices. Visionisti’s platform is ideal to be incorporated into a sterile, pre-assembled, pre-adjusted package as part of a drug-device combination product.
A MODERN OPHTHALMIC DELIVERY SYSTEM
An effective system for drug delivery to the back of the eye should embody four general characteristics.6 A successful suprachoroidal delivery system has the ability to meet all of these criteria, and Visionisti’s platform achieves just that (Figure 5).
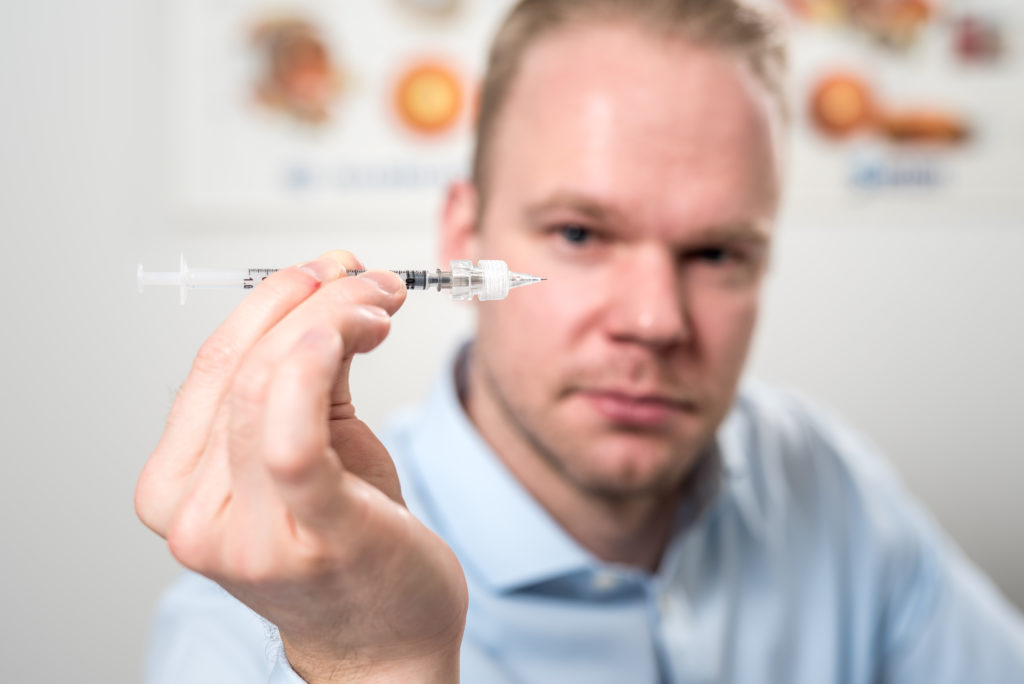
Figure 5: Visionisti’s suprachoroidal injection platform is minimally invasive and safe, well targeted to the desired tissue, capable of sustained delivery and simple to use (image credit to Elias Jokiranta Photography).
Minimally Invasive and Safe
Suprachoroidal injection using Visionisti’s platform takes place as a standardised procedure within the administering clinician’s office, consisting of a perpendicular transscleral injection without the need for a surgical operation or invasive cannulation of the eye. In particular, the needle guide enhances the consistency safety of the procedure by increasing the stability of the eye and making the process simpler and readily reproducible, reducing the necessary skill level required to administer the injection.
Well Targeted to the Desired Tissues
Suprachoroidal injection provides direct access to the site of therapeutic action (choroid and retina) and avoids the inner limiting membrane barrier. Additionally, the delivery route has been shown to maintain higher drug concentrations and increased bioavailability of therapeutics at the target site for longer periods of time, while also compartmentalising the administered drug to the desired target sites. Reduced drug exposure in undesired regions of the eye leads to fewer side effects and complications and means that the therapy does not affect the eye’s optical axis.
Capable of Sustained Delivery
In order to reduce how frequently patients must receive injections to treat their ophthalmic conditions, it is desirable for drug product to remain available to the target site after the injection takes place. While still a subject of investigation, studies have shown that some drugs, such as triamcinolone acetonide, may reside in the eye for a significantly longer period when delivered into the suprachoroidal space compared with when delivered by intravitreal injection.7 This, coupled with the higher bioavailability to the choroid and retina, means that suprachoroidal delivery has the potential to demand a less frequent dosing regimen, which may in turn reduce the burdens on both patients and treatment providers, improving quality of life for all parties.
Simple
Visionisti’s platform is a convenient, easy-to-use all-in-one tool. It stabilises the eye and keeps the eyelid open, making the injection process simpler to perform and more comfortable for the patient. The platform standardises the injection procedure, making it repeatable, faster and easier for the administering clinician. In a similar way, the needle guide reduces potential user error by ensuring that injections are performed in a predefined way.
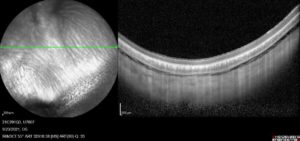
Figure 6: An IR image (left) and OCT cross-section (right) of a rabbit eye prior to injection into the suprachoroidal space.
PRECLINICAL STUDIES
Visionisti’s platform has successfully undergone both ex vivo and in vivo preclinical trials. A recent trial conducted by Absorption Systems California (CA, US) tested the feasibility of the system for suprachoroidal injection into rabbits. Prior to any injection, the eyes were imaged using fluorescein angiography (FA), infrared (IR) and optical coherence tomography (OCT). These baseline images showed a fully collapsed suprachoroidal space (Figure 6). After the baseline was taken, a 100 μL injection was delivered to the suprachoroidal space using a 30G needle with Visionisti’s platform adapting it for suprachoroidal use. A second set of images was then taken, with the cross-sections taken by OCT clearly showing an opening of the suprachoroidal space (Figure 7).
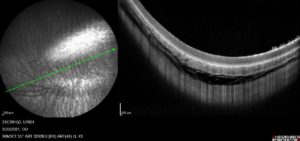
Figure 7: An IR image (left) and OCT cross-section (right) of a rabbit eye after a 100 μL injection into the suprachoroidal space using a 30G needle adapted using Visionisti’s platform. The red arrow indicates the post-injection opening of the suprachoroidal space.
FUTURE OUTLOOK
Further to its suprachoroidal application, research suggests that this technology has the potential to deliver to the supraciliary space,8 subretinal space9 and cornea.10 One area of interest is in delivering an in-situ-forming hydrogel to the suprachoroidal space, which may reduce intraocular pressure in glaucoma patients.11 An expansion of the potential applications of microneedles means a similarly expanded potential for Visionisti’s platform to enable standard hypodermic and solid needles to be used for delivering injectable therapies to these sites. Furthermore, one added benefit of Visionisti’s platform over specialised microneedles is that the same adjustable adapter can be used to tailor the exposed needle length to each of the potential delivery targets, rather than requiring different microneedles specifically suited to each. The intellectual property rights of Visionisti’s platform are widely protected; Visionisti has technology patents in Europe, Japan and the US, as well as design patents in Europe and the US.
In summary, suprachoroidal delivery has the potential to be a major step up in drug delivery to the choroid and retina, which represents a rapidly growing sector of the market. Visionisti’s platform fulfils all the requirements of a modern system with a simple, all-in-one tool that provides a safer, more effective and more convenient way to deliver drugs to the back of the eye for all stakeholders. This technology represents a rare opportunity to advance the field of drug delivery and may provide a better quality of life to patients and physicians.
REFERENCES
- Barakat MR, “Suprachoroidal Injection Update”. Retinal Physician, Sep 2019.
- Hancock SE et al, “Biomechanics of Suprachoroidal Drug Delivery: From Benchtop to Clinical Investigation in Ocular Therapies”. Expert Opin Drug Deliv, Jun 2021, Vol 18(6), pp 777–788.
- Wan C et al, “Suprachoroidal Delivery of Small Molecules, Nanoparticles, Gene and Cell Therapies for Ocular Diseases”. Pharmaceutics, Feb 2021, Vol 13(2), p 288.
- Chiang B, Jung JH, Prausnitz MR, “The Suprachoroidal Space as a Route of Administration To the Posterior Segment of the Eye”. Adv Drug Deliv Rev, Feb 2018, Vol 126, pp 58–66.
- Patel SR et al, “Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles”. Pharm Res, Jan 2011, Vol 28(1), pp 166–176.
- Holekamp N, Demko S, “Suprachoroidal Delivery ‘Brings Gene Therapy Into the Office Setting’ for Wet AMD”. Healio News, Nov 2021.
- Emami-Naeini P, Yiu G, “Medical and Surgical Applications for the Suprachoroidal Space”. Int Ophthalmol Clin, 2019, Vol 59(1), pp 195–207.
- Kim YC, Edelhauser HF, Prausnitz MR, “Targeted Delivery of Antiglaucoma Drugs to the Supraciliary Space Using Microneedles”. Invest Ophthalmol Vis Sci, Sep 2014, Vol 55(11), pp 7387–7397.
- Yiu G et al, “Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates”. Mol Ther – Methods Clin Dev, Mar 2020, Vol 16, pp 179–191.
- Kim YC et al, “Intrastromal Delivery of Bevacizumab Using Microneedles to Treat Corneal Neovascularization”. Invest Ophthalmol Vis Sci, Sep 2014, Vol 55(11), pp 7376–7386.
- Chae JJ et al, “Drug-Free, Nonsurgical Reduction of Intraocular Pressure for Four Months after Suprachoroidal Injection of Hyaluronic Acid Hydrogel”. Adv Sci (Weinh), Jan 2021, Vol 8(2), 2001908.

