To Issue 137
Citation: Mayer T, “Accelerating Development of Subcutaneous Delivery of Large-Volume Biologics”. ONdrugDelivery, Issue 137 (Sep 2022), pp 52–56.
Tom Mayer discusses how Sonceboz’s vial-based on-body injector – the LVI-V – can expedite the launch of large-volume SC delivery of biologics by leveraging existing primary container technology, reducing associated risks and total cost of ownership.
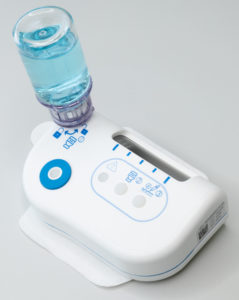
Figure 1: The large-volume injector.
The pharma industry needs a cost-effective plan of action that makes the subcutaneous (SC) delivery of large-volume biologics possible in the near future. Sonceboz believes its LVI-V is the gateway to that future.
The LVI-V is an on-body injector (OBI) that connects to vials, meaning that, in its standard configuration, it can deliver a high bandwidth of volumes, ranging from 1–20 mL (Figure 1). It has a programmable electromechanical pump system capable of delivering drugs at various speeds across a wide range of viscosities while providing accurate and consistent drug delivery. Furthermore, its sensors provide feedback to the user to ensure that the intended dose is delivered. It is part of a platform of products that Sonceboz is developing to seamlessly integrate into current infrastructure, providing pharma companies with new, adaptable tools for complete lifecycle management.
“The value proposition for OBIs is clear: the ability to deliver larger doses of injectable drugs via the SC route.”
THE CASE FOR VIAL-BASED ON-BODY INJECTORS NOW
The value proposition for OBIs is clear: the ability to deliver larger doses of injectable drugs via the SC route. OBIs (sometimes called wearable injectors, patch pumps or bolus injectors) are being designed to store volumes typically greater than 3 mL.1 As there is a limit to the time a patient can hold an injection device in place during administration, pen injectors and prefilled syringes are not ideal for drugs that require volumes larger than 3 mL (and therefore require delivery over a longer duration).2
Device companies have introduced many different technologies into the injector space over the last 10 years. However, an independent observer may have noticed the limited number of launches for OBI devices. The OBIs currently available on the market can only hold small volumes in the 3–5 mL range. It may seem that the technology, especially for injection volumes greater than 5 mL, lacks maturity and is still in the developmental phase.
Indeed, progress has been stalled by the fact that many designers of OBIs promote the design of custom primary drug containers, which they suggest would improve the ease of use for patients — a feature that is high on the list of requirements for OBIs.1 The primary container is a crucial component of an OBI because it is the main point of contact between the drug and the device;2 as such, both the device and the custom primary container must be validated after development to ensure drug integrity and stability throughout the intended shelf life of the drug product.
The primary container (which may contain stoppers, closures, plungers and/or lubricants, such as silicone oil) comes in direct contact with the drug, potentially leading to interactions between the container materials and the complex molecular structure of long-chain biologics, which may then reduce the efficacy of these biologics. Extensive risk analyses and stability tests are required before regulatory approval.2 In most cases, fully validated internal – or even external – fill-and-finish solutions do not yet exist.
“Too much focus on custom primary containers is a potential distraction; instead, pharma and biotech companies can leverage existing and proven primary container technology.”
Thus, the development of custom large-volume containers is ongoing and will likely take a while to be ready. Meanwhile, pharma companies seek a solution that will not derail a product filing and launch timelines. Too much focus on custom primary containers is a potential distraction; instead, pharma and biotech companies can leverage existing and proven primary container technology, freeing up resources to focus on other aspects of drug development, while at the same time reducing overall project risks.
LEVERAGING EXISTING PRIMARY CONTAINER TECHNOLOGY
Sonceboz is developing a solution that will seamlessly integrate into the pharma industry’s current infrastructure. Its electromechanical OBI platform makes it possible to connect to various existing primary drug containers of capacities up to 20 mL, or even higher, such as vials or cartridges — without changing the overall system architecture of the OBI.
To allow maximum flexibility, the large-volume delivery platform comes in different variants (Figure 2), including:
- The vial-connecting injector (LVI-V)
- The prefilled and preassembled injector (LVI-P)
- The prefilled injector that is assembled by the user at the point of use (LVI-U)
- The dual-cartridge injector (DCI)
- The auto-reconstitution injector (ARI).
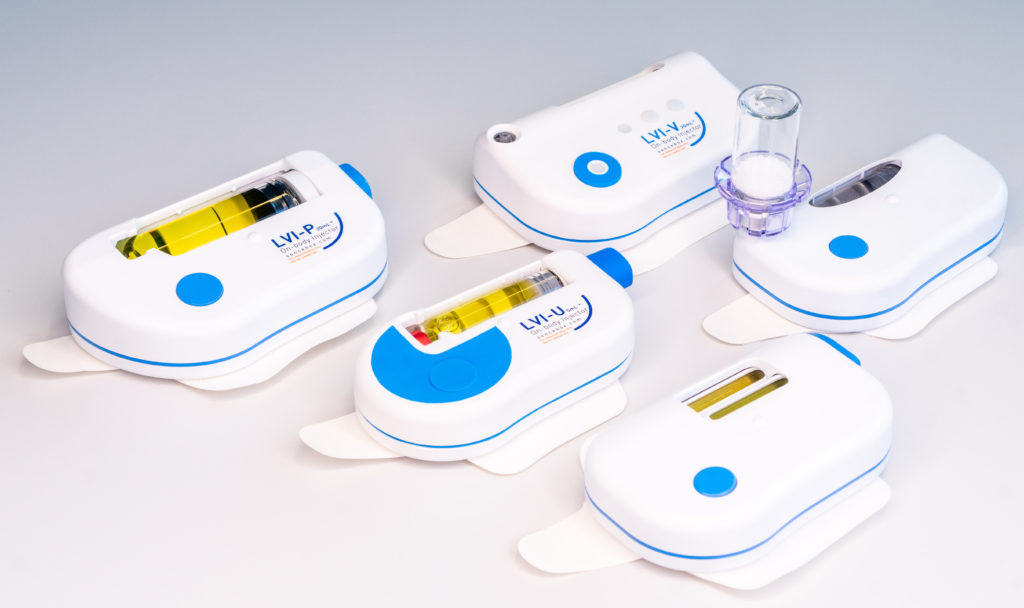
Figure 2: The Sonceboz wearable injector platform offers different delivery volumes, programmable pump delivery profiles and container options.
In the case of Sonceboz’s dual-cartridge injector, the same architecture can connect and independently inject from two existing primary containers, either simultaneously or sequentially. Its auto-reconstitution injector will automatically reconstitute lyophilised drug products stored in existing primary containers. Various handheld injectors can reconstitute lyophilised drugs, but it is an unusual feature for OBIs.1
The company’s most advanced OBI is the vial-connecting injector, LVI-V. Most pharma companies and contract manufacturing organisations have vial-filling capabilities. Vials are easier to adopt from a drug stability and compatibility perspective, since 90–95% of liquid drug formulations already rely on vials as the primary container.
In contrast to electromechanical systems, strictly mechanical systems require a substantial redesign to both the device and the drive system whenever there is a change to the format or size of the primary container. However, because the format or size of the primary container can evolve throughout a product’s lifecycle, it is easier to use an electromechanical device with a pump system independent of the drug container, as such a device would only require software adaptations.
Cost effectiveness is the first advantage of an electromechanical OBI platform that can connect to various existing primary drug containers. The smaller patient populations for biologics mean that cost effectiveness is already a top priority for manufacturers to fill these small quantities.2 Considering that custom primary containers require custom fill-and-finish services,2 manufacturers would also have to factor in the cost of these services as part of a total cost of ownership (TCO) evaluation.
Furthermore, an electromechanical pump helps to reduce dead volume because the system uses a vacuum to maximise the volume of drug delivered per container to the patient. Sonceboz stringently follows design-for-manufacture and design-to-cost principles, ensuring Swiss quality at competitive prices from a TCO perspective. The company believes that highly integrated platform design leads to better cost efficiency.
Accelerating time to market is the second advantage of these systems. By offering different delivery volumes, programmable pump delivery profiles and container options, pharma companies can easily leverage Sonceboz’s platform across multiple drug products, as well as across a single product’s lifecycle – from clinical trials (where final dosing often has yet to be determined) to commercial distribution – thereby taking a level of risk out of development.
Patent protection is another advantage. As the pace of competition increases for biologics, originator products are losing market share to biosimilars. Developers of originator intravenous (IV) drugs can adopt an IV-to-SC drug repositioning to maintain patent protection.
“The key to successfully implementing self-administration of biologics via large-volume OBIs is avoiding potential user errors during drug and device handling.”
IMPROVING DRUG ACCESS BY DECENTRALISING CARE
There is an ongoing need for a simple device that creates new possibilities for administering drug therapies and puts an end to the requirement for patients to visit large healthcare centres. Those new possibilities could include the home, the family doctor’s office or the injection clinic.
Healthcare professionals who are active in treating inflammatory bowel disease and patients with this chronic disease exchanged their experiences with SC delivery of treatment in a recent virtual community meeting, revealing that its benefits include self-administration by the patient and shorter application time with fewer infusion-related adverse events – consequently lowering healthcare costs. For patients, being able to administer their treatments at home leads to an improvement in their quality of life and reduced need to travel to a healthcare institution – consequently reducing costs for the patient.3
Hospitals carry the connotation of serious illness.4 Typically, patients prefer SC over IV administration, according to a systematic literature review of studies including patients with chronic immune system disorders. The same study concluded that patients preferring SC administration tended also to prefer at-home treatment because it offers the convenience and comfort of being at home and avoids having to attend hospital.5
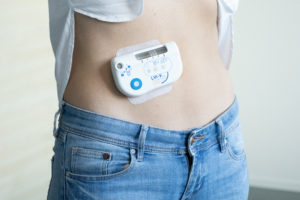
Figure 3: The large-volume injector on a patient’s body.
The key to successfully implementing self-administration of biologics via large-volume OBIs is avoiding potential user errors during drug and device handling. Sonceboz makes device use straightforward: after connecting the drug container to the device via an adapter, the user places the device on the skin (Figure 3) and activates it by touching a single button, as they would an autoinjector. The device will take care of placing the injection cannula and initiating the injection with embedded sensors that monitor these processes.
Users will have full mobility during the injection. The device’s orientation while users move around will not affect the treatment delivery because the drug is first transferred from the vial (a non-collapsible container) to a collapsible container (the internal reservoir). While this adds an assembly step, the design ensures that the pump can access the drug regardless of the device’s or patient’s spatial orientations. This is important to ensure that the intended dose is safely administered. Sonceboz’s data suggest compatibility of the GentleTouch™ pump design with large-molecule drugs; both the molecule integrity and activity remain intact. Furthermore, the design is completely free of silicone oil. Some biologic molecules are not compatible with silicone oil, which is often used to lubricate gliding systems in mechanical pumps.
Of course, usability improves with feedback. The Sonceboz OBI platform comes with built-in connectivity using Bluetooth Low Energy and near-field communication (NFC) technology, enabling digital health applications. In addition to transferring data to the patient’s healthcare professional, the device can provide feedback, such as confirmation of when the injection is completed.
Consistency is also vital to building confidence in successfully implementing self-administration of biologics via large-volume OBIs. An electromechanical system allows precise injection rate control across the total container volume. Regulators require that the intended dose indicated on the drug label is delivered regardless of the patient or variation in the injection conditions. The designer knows the device’s fluid path and mechanical powerpack and can revise the dimensions accordingly. Sonceboz’s electromechanical drive system enables load monitoring and helps achieve flow accuracy of about ±2%.
Finally, patients must be comfortable using the device, physically (in terms of comfort with the needle insertion) and socially (in terms of comfort administering a treatment in public). This is helped by the fact that SC injections are less invasive than intravenous infusions. To prevent needlestick injuries, the dynamic needle-insertion system (DNIS) uses a solid needle in combination with a soft cannula made from polytetrafluoroethylene (PTFE). The DNIS releases the needle and then retracts, leaving behind only the soft cannula for optimal patient comfort. The device is palm sized and small enough to be disguised under clothing. Because Sonceboz uses high-quality motors and leverages its high-volume automotive production for assembly, the device emits only a low level of noise.
PREPARING FOR A SUSTAINABILITY-MINDFUL FUTURE
Sonceboz applies, whenever possible, the four “R”s (reduce, reuse, replace, recycle) during the entire lifecycle of its OBI device, especially during design and manufacturing (Figure 4):
- Reduce the carbon footprint as much as possible
- Reuse the same material as much as possible for several components
- Replace and scrap non-conforming components by checking every single part throughout the assembly process
- Recycle by creating an efficient collection network and sorting components from used devices.
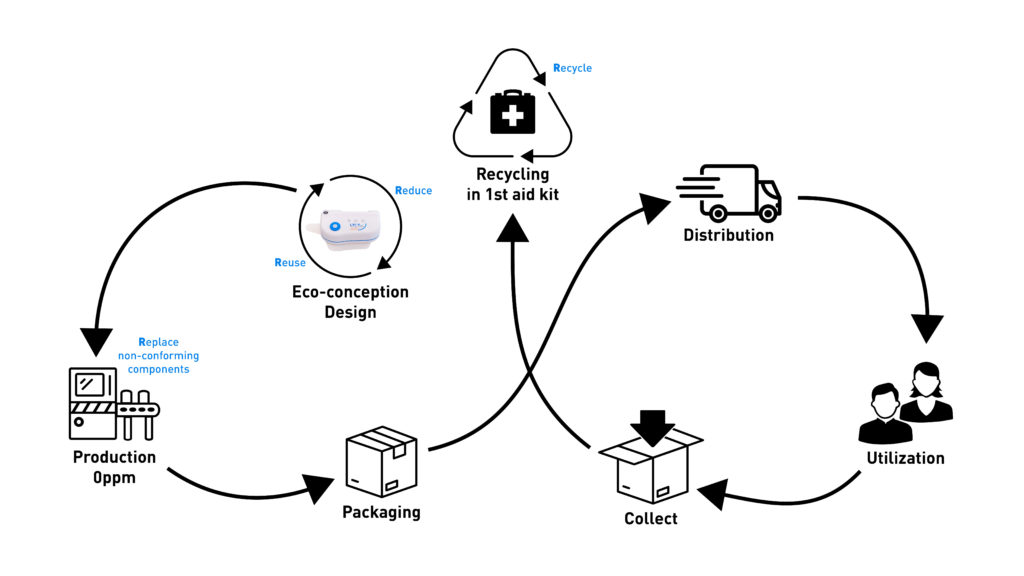
Figure 4: Sonceboz applies, whenever possible, the four “R”s (reduce, reuse, replace, recycle) during the entire lifecycle of its OBI device, especially during design and manufacturing.
Just because something is “recyclable”, it does not ensure it will be recycled. Sonceboz is actively investigating the end-of-life stage of its OBIs and determining any possible second life for used materials. While sustainability is an effort to reduce the impact on the environment, patient safety cannot be compromised; reusing parts from disposable devices is therefore challenging due to the validation process. Recycling components into other medical devices might not be feasible, but transforming parts into other tools, such as first-aid kits, may be an interesting alternative.
Additionally, since the OBI will enable home treatment, thereby reducing patient travel, it will dramatically reduce the environmental impact while minimising the exposure patients will have to hospital-acquired infections.
DESIGN AND LEADING THE WAY INTO THE FUTURE
Sonceboz has considered many, if not all, aspects of its OBI design, from cost effectiveness to scalability and usability to patient comfort and safety. The design complies with the standards in ISO 11608-6, “Needle-based injection systems for medical use – Requirements and test methods – Part 6: On-body delivery systems.” Sonceboz is now partnering with pharma companies to prepare its devices for clinical use. It anticipates that the next generation of devices will be prefilled and preloaded once the development of custom drug containers has been tested to the full extent according to ISO standards. Until then, the LVI-V can lead the way to accelerate drug development, extend patent protection and facilitate at-home drug administration in a cost-effective and sustainability-mindful fashion.
REFERENCES
- Oakley T, “Wearable Injectors, Latest Devices and Recent Trends”. ONdrugDelivery, Issue 111 (Sep 2020), pp 6–9.
- Bauert D, Kumar Busimi A, “Expert View: Primary Packaging for Wearable Injectors”. ONdrugDelivery, Issue 100 (Sep 2019), pp 26–28.
- Jonaitis L, Markovic S, Farkas K et al, “Intravenous versus subcutaneous delivery of biotherapeutics in IBD: an expert’s and patient’s perspective”. BMC Proc, 2021, Vol 15, Article 25.
- “False claim: Hospital stands for house of sick people in trauma and labor”. Reuters, Apr 2020.
- Overton P et al, “Patient Preferences for Subcutaneous versus Intravenous Administration of Treatment for Chronic Immune System Disorders: A Systematic Review”. Patient Prefer Adherence, 2021, Vol 15, pp 811–834.

